Health Effects of Vaping: Virtual Collaborative Forum
What We Heard Report
Date: April 26, 2022
Time: 2:00 p.m. to 4:00 p.m. (EDT)
Table of Contents
- Introduction
- Workshop Objectives
- Message from the Scientific Director
- Agenda
- Facilitators
- Session 1: Challenges & Mitigation Strategies
- Session 2: Next Steps and Opportunities for Collaboration
- Project Updates: Youth vaping of nicotine, cannabinoid extracts, THC, and other compounds
- Project Updates: Short and/or long-term health effects of vaping (including lung injury in youth and/or adults)
- Project Updates: Behaviours and associated health and social impacts of vaping product use (youth and/or adults)
- Project Updates: Mental health, addiction and dependence in the context of vaping (youth and/or adults)
- Project Updates: Vaping policy issues related to youth and/or adults
- Project Updates: Other areas related to the health consequences of vaping in youth and/or adults
- Appendix A – Whiteboard: Research Related Challenges and Mitigation Strategies
- Appendix B – Whiteboard: Next Steps and Opportunities for Collaboration
Introduction
The Health Effects of Vaping Collaborative Forum (Forum) event was co-hosted by the Canadian Institutes of Health Research (CIHR) Institute of Circulatory and Respiratory Health (ICRH), in partnership with the Canadian Cancer Society (CCS), on April 26th, 2022, 2:00 p.m. – 4:00 p.m. EDT.
The Forum was developed in response to an identified need by investigators funded in the Health Effects of Vaping Catalyst Grant funding opportunity. This funding opportunity represented a partnership among the CCS, CIHR, ICRH and the Institutes of Cancer Research (ICR), Human Development Child and Youth Health (IHDCYH), and Neurosciences Mental Health and Addiction (INMHA). This voluntary Forum addressed the many challenges faced by the 27 funded research teams due to the restrictions imposed during the COVID-19 pandemic. As such, the objectives of this researcher-led Forum were to enable grantees to connect and network with colleagues, as well as share their successes, challenges and discuss future opportunities for collaboration.
The virtual Forum hosted 63 participants from across Canada over a two-hour Microsoft Teams event. The participants included members from the 27 funded research teams, collaborating CIHR Institutes, external partners (CCS) and other key organizations (National Institutes of Health - National Heart, Lung, and Blood Institute).
The Forum opened with a welcome message from the event Chair, Dr. Brian H. Rowe, Scientific Director of ICRH. The event consisted of two breakout sessions which were each followed by a report back session. For the breakout sessions, participants were separated into four smaller virtual breakout rooms to discuss one of the identified session themes:
- research related challenges and mitigation strategies; and,
- next steps and opportunities for collaboration.
The breakout room discussions were moderated by four volunteer Nominated Principal Investigators (NPIs) representing four different funded research teams. These discussions were facilitated using Jamboard –a collaborative digital whiteboard application (refer to Appendix A & B)– to permit the visual thematization of issues discussed within the identified session theme. After a session theme was discussed, participants were brought back together for a collective discussion during the report back session. During the report back sessions, each breakout room moderator was allocated three-minutes to provide an overview of key points discussed in their respective breakout room. The report-back discussion was facilitated by Dr. Ryan Perry, Associate Scientific Director of ICRH, and graphically recorded by Fuselight Creative artist Tanya Gadsby. The graphic recordings provided a high-level visualization of ideas in real-time. The visual tools (Jamboard and graphic recording) permitted iterative discussion and facilitated the virtual collaboration amongst the research teams and Forum participants.
Workshop Objectives
The aim of the Forum was to support collaboration and information exchange among research teams funded through the Health Effects of Vaping Catalyst Grant funding opportunity. During this researcher-led virtual event, participants took part in moderated discussions on:
- research-related challenges and mitigations strategies; and
- next steps and opportunities for collaboration.
Message from the Scientific Director
Vaping in Canada has become a popular activity, especially in youth populations; however, the health effects of vaping are largely unknown. Mobilizing the research community and stakeholders to respond to increasing concerns regarding these evidence gaps related to vaping on the health of Canadians, was a key driver for launching the Health Effects of Vaping (Vaping) catalyst grant competition. At the time (pre-pandemic), there was a need to expand and strengthen our overall knowledge base related to vaping, particularly in the areas of:
- Youth vaping;
- The health effects of vaping (including E-cigarettes, or Vaping, product use Associated Lung Injury {EVALI} and other lung injury) in youth and/or adults;
- Vaping habits and behaviours (in youth and/or adults); and
- Mental health and addiction in the context of vaping in youth and/or adults.
The CIHR pause on funding in March 2020 in response to the novel coronavirus pandemic was lifted for the Vaping catalyst funding competition in June 2020. This reflected the added urgency to understand any link(s) between vaping and severe adverse outcomes of COVID-19 as the severe acute COVID-19 infection predominately affects the lungs. The overall objective of the funding opportunity was to support the generation of relevant evidence through rigorous scientific methods in key research areas related to vaping in both youth and adults. This one year of funding was designed to generate preliminary observations, data or knowledge; support projects that have the potential to generate high impact results and/or were innovative in approach; and facilitate consideration and application of new evidence to inform ongoing and future development of policies, practices and programs related to vaping.
With the various social, physical and health countermeasures introduced to mitigate COVID-19 infections in Canada, these also introduced challenges, barriers and in some instances, opportunities for health research. This has understandably resulted in research project delays, which have extended the time frame of the vaping catalyst grants. Moreover, this has delayed the planned end-of-grant workshop to facilitate knowledge translation among stakeholders and researchers.
In the interim, the Health Effects of Vaping: Virtual Collaborative Forum was developed in response to interest expressed by investigators funded in the vaping catalyst grant competition for an opportunity to share, collaborate and network with colleagues on successful strategies that have facilitated research progress during the pandemic. In addition, investigators wanted to identify opportunities for future collaborations as research projects advance to completion.
I encourage you to take the opportunity to actively participate in discussions, share your experiences and identify opportunities to amplify, leverage or advance your research. I look foreword to your contributions and participation as we move forward to address knowledge gaps in this important research area. Finally, we wish to prepare for a broader engagement of practice, policy and decision-maker groups at the end-of-grant workshop.
Brian H. Rowe, MD, MSc, CCFP(EM), FCFP, FCCP, FCAHS
Scientific Director, CIHR Institute of Circulatory and Respiratory Health (CIHR-ICRH)
Professor, Department of Emergency Medicine and School of Public Health
University of Alberta
Agenda
April 26, 2022, 2:00 - 4:00 p.m. EDT
| Time (EDT) | Item | Speakers |
|---|---|---|
| 2:00 p.m. to 2:10 p.m. | Welcome and Introductions | Brian H. Rowe |
| 2:10 p.m. to 2:15 pm | Setting the Stage | Brian H. Rowe |
| 2:15 p.m. to 2:35 p.m. | Breakout Session 1: Research related challenges and mitigation strategies | Moderators: Michael Chaiton Jibran Khokhar Donald Sin Ajitha Thanabalasuriar |
| 2:35 p.m. to 3:05 p.m. | Group Discussion: Research related challenges and mitigation strategies Fuselight Creative Graphic Recording |
Moderators: Michael Chaiton Jibran Khokhar Donald Sin Ajitha Thanabalasuriar Ryan Perry Fuselight Creative |
| 3:05 p.m. to 3:25 p.m. | Breakout Session 2: Next steps and opportunities for collaboration | Moderators: Michael Chaiton Jibran Khokhar Donald Sin Ajitha Thanabalasuriar |
| 3:25 p.m. to 3:55 p.m. | Group Discussion 2: Next steps and opportunities for collaboration Fuselight Creative Graphic Recording |
Moderators: Michael Chaiton Jibran Khokhar Donald Sin Ajitha Thanabalasuriar Ryan Perry Fuselight Creative |
| 3:55 p.m. to 4:00 p.m. | Summary and Closing Remarks | Brian H. Rowe |
Facilitators
Meeting Chair
Brian H. Rowe
Scientific Director, CIHR Institute of Circulatory and Respiratory Health
Moderators
- Michael Chaiton
Independent Scientist
Centre for Addiction and Mental Health - Jibran Khokhar
Assistant Professor
University of Guelph - Ryan Perry
Associate Scientific Director
CIHR Institute of Circulatory and Respiratory Health - Donald Sin
Professor
University of British Columbia - Ajitha Thanabalasuriar
Assistant Professor
McGill University
Session 1: Challenges & Mitigation Strategies
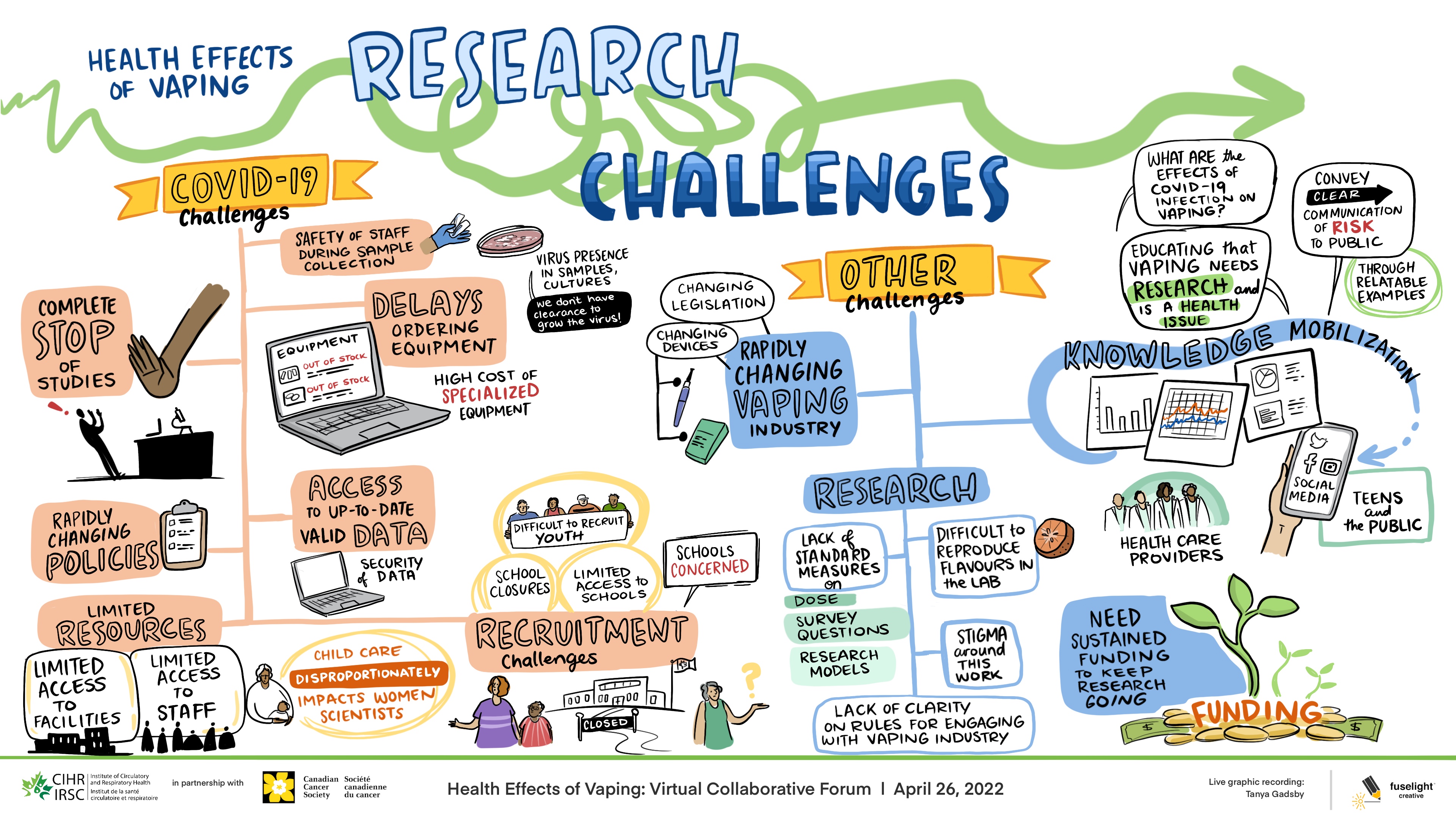
Long Description
Health Effects of Vaping: Research Challenges
- COVID-19 Challenges
- Safety of staffduring samplecollection
- Virus presence in samples, cultures
- We don't have clearance to grow virus
- Delays ordering equipment
- Equipment
- Out of stock
- High cost of specialized equipment
- Complete stop of studies
- Access to up-to-date valid data
- Security of data
- Rapidly changing policies
- Limited resources
- Limited access to facilities
- Limited access to staff
- Child care disproportionately impacts women scientists
- Difficult to recruit youth
- School closures
- Limited access to schools
- Schools concerned
- Recruitment challenges
- Closed
- Other challenges
- Changing legislation
- Changing devices
- Rapidly changing vaping industry
- Research
- Lack of standard measures
- Difficult to reproduce flavours in the lab
- Dose
- Survey questions
- Research models
- Stigma around this work
- Lack of clarity on rules for engaging with vaping industry
- What are the effects of COVID-19 infection on vaping?
- Educating that vaping needs research and is a health issue
- Conveyclear communication of risk to public
- Through RelatableExamples
- Knowledge mobilization
- SocialMedia
- Teens and the public
- Health care providers
- Need sustained funding to keep research going
- Funding
Summary
This session included four breakout rooms where Forum participants had 20 minutes to discuss the challenges and mitigation strategies related to progress of their vaping research projects in the context of the COVID-19 pandemic. Please see Appendix A for whiteboard affinity mapping. Participants were then brought back together to hear three-minute overviews of the breakout room discussions provided by the moderators. A 30 minute large group discussion then occurred following the three-minute overviews.
The research teams discussed COVID-19 related challenges concerning: 1) research delays due to the pause of non-COVID-19 focused research studies; 2) resource limitations due to limited availability of equipment, facilities and staff; 3) study recruitment challenges; 4) limited access to up-to-date reliable data; 5) ensuring safe work environments for sample collection; and, 6) rapidly changing policy environment.
Other non-COVID-19 related challenges were also discussed. They included the need for: 1) improved knowledge mobilization to clearly convey the risks of vaping to the public; and, 2) sustained funding to support research on vaping. Participants also discussed challenges specific to conducting vaping research, including: 1) the lack of standard measures and data collection tools (i.e. survey questions); 2) diversity of biomedical research models; and, 3) reproducibility of pre-clinical vape delivery systems. Stigma associated with conducting vaping research also created challenges for teams in areas such as funding and recruitment. Finally, the rapidly changing vaping product industry also posed challenges due to evolving device types, flavours as well as technology challenges related to vape coils, wicks and atomizers. See Figure 1 for the graphic recording by Fuselight Creative.
Session 2: Next Steps and Opportunities for Collaboration
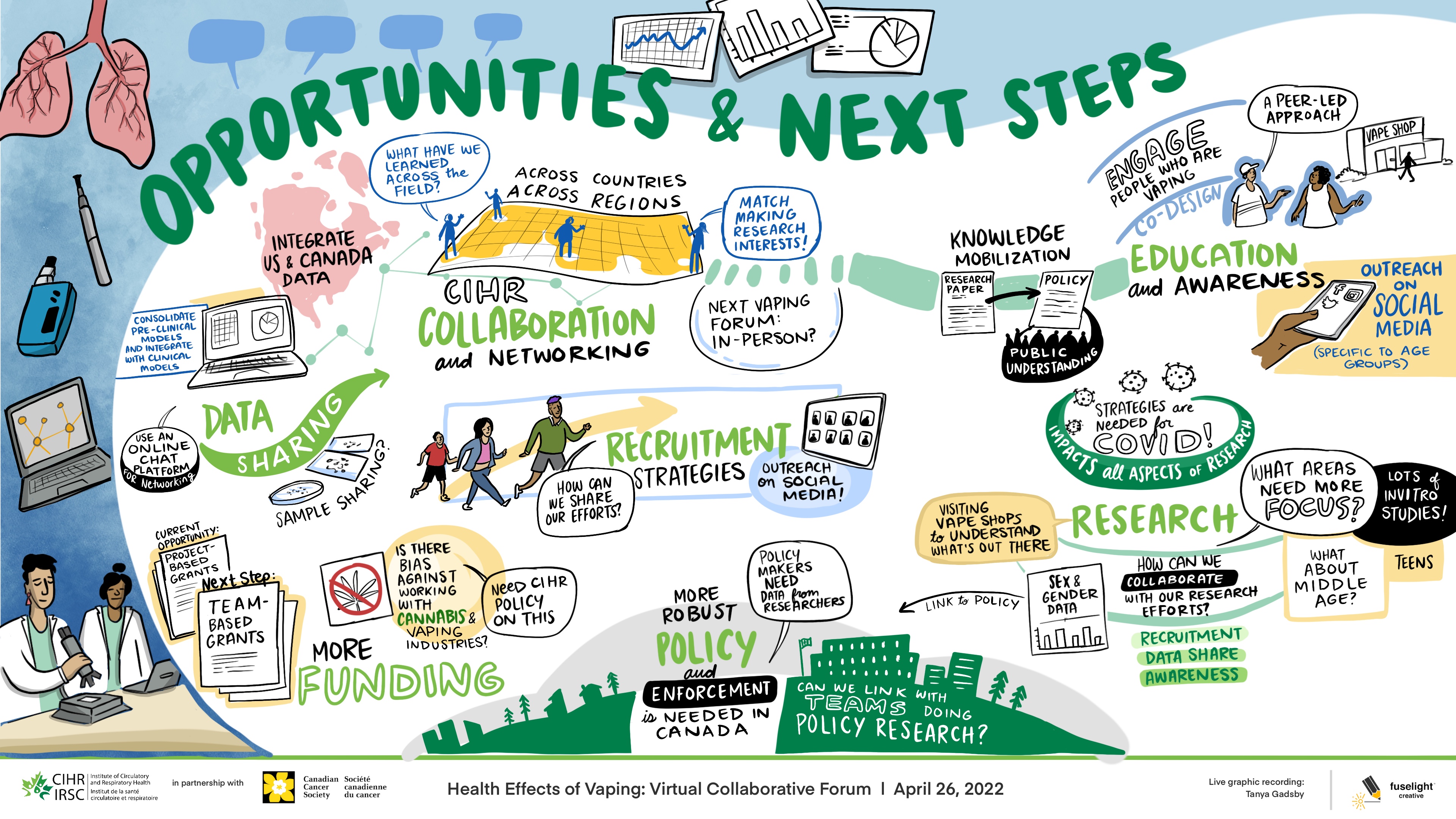
Long Description
Opportunities & Next Steps
- Consolidate pre-clinical models and integrate with clinical models
- Use an online chat platform for networking
- Current Opportunity
- Project-Based Grants
- Next Step
- Team-Based Grants
- Integrate US & Canada Data
- Data Sharing
- Sample Sharing?
- More Funding
- Is there bias against working with Cannabis & Vaping Industries – Need CIHR policy on this
- What have we learned across the field
- CIHR Collaboration and Networking
- Across Countries
- Across Regions
- Match Making Research Interests
- Next Vaping Forum: In-person?
- How can we share our efforts?
- Recruitment Strategies
- Outreach on social media
- More robust policy and enforcement is needed in Canada
- Policy makers need data from researchers
- Can we link with teams doing policy research?
- Knowledge mobilization
- Research Paper
- Policy
- Public understanding
- Visiting vape shops to understand what's out there
- Research
- What areas need more focus?
- Lots of in vitro studies
- Link to policy
- Sex & gender data
- How can we collaborate with our research efforts?
- Recruitment
- Data Share
- Awareness
- What about middle age?
- Teens
- Engage people who are vaping
- Co-design
- A peer-led approach
- Vape Shop
- Education and awareness
- Outreach on social media (specific to age groups)
- Strategies are needed for COVID!
- Impacts all aspects of research
- Live graphic recording:
- In partnership with
Summary
This second session was identical in format; however, it focused on next steps and opportunities for collaboration. See Appendix B for whiteboard affinity mapping. Groups rejoined the main virtual meeting room and participants listened to the three-minute overviews provided by the moderators of each breakout room discussion, after which a broader 30-minute group conversation ensued.
Participants discussed opportunities for: 1) fostering collaboration among countries and regions through matching research interests amongst the research teams; and, 2) data-sharing between Canada and the United States to support the access to valid and relevant data. Participants expressed that a collaborative initiative would reduce research redundancy, promote a more efficient use of funds, support study recruitment and help to address the current gaps in vaping research. Teams also discussed knowledge mobilization, expressing that the engagement with people with lived and living experience (i.e., people who vape), regulators and policy groups were important to promote education, awareness and initiate change in the area to address the health effects of vaping. There seemed to be consensus on the idea that building relationships with people who are vaping, regulators and policy groups would support a peer-led approach to better inform policy and the public through co-designed and data driven education and awareness. The role of industry in vaping research activities was also discussed and efforts to meaningfully address conflict of interest challenges when engaging with industry were proposed. See Figure 2 for the graphic recording by Fuselight Creative.
Project Updates: Youth vaping of nicotine, cannabinoid extracts, THC, and other compounds
-
Cannabis vaping experiences and decisions among youth and young adults in Manitoba (The CaVED Project)
Nominated Principal Investigator
Lynda Balneaves
Associate Professor, College of Nursing, University of Manitoba
Lynda.Balneaves@umanitoba.caPrincipal Investigators
- Shelley Turner
- Roberta L Woodgate
Knowledge Users
- Katarina Kolar
Health Canada - Neil Johnston
Manitoba Lung Association - Matt Henderson
Seven Oaks School Division - Cheryl Cusack
Manitoba Government, Population and Public Health Branch
Co-investigators
- David G Hammond
- Nathan C Nickel
Project Summary
After some initial delays as the result of the COVID-19 pandemic, the CaVED project was launched in early 2022. A provincial survey of cannabis- and vaping-related behaviour, knowledge and beliefs among youth and young adults is currently underway in Manitoba, with over 900 surveys completed to date.
The data collected will provide important insights regarding patterns of cannabis use and vaping, knowledge and beliefs regarding the potential harms and benefits of cannabis use and vaping, and the intersection between cannabis vaping and the use of other substances within this population. Preliminary results suggest nearly 70% of the sample have used cannabis, with 86% of those individuals using a vaping device to inhale cannabis. The top three reasons for using cannabis includes using it to relax, to help with sleep, and to help cope with stress. Overwhelmingly, individuals indicated that compared to other methods of taking cannabis, vaping is easier to hide. The survey will continue until the end of April 2022, when in-depth qualitative interviews will begin with a diverse sample of youth and young adults drawn from participants who expressed interest in sharing more of their experiences and decision-making process related to cannabis and vaping.
Data collection is anticipated to end in June 2022, with analysis completed by September 2022. Dissemination activities, in collaboration with the CaVED Youth Advisory Committee, will continue until December 2022.
-
Teens talk vaping: A qualitative integrated knowledge translation study to co-produce vaping research and educational tools with teens
Nominated Principal Investigator
Jason Gilliland
Professor, University of Western Ontario
jgillila@uwo.caPrincipal Investigator
Stephanie Coen
Knowledge User
Terry Spencer
Research and Evaluation Officer
London District Catholic School BoardCo-investigators
- Anita G Cramp
- Christopher Mackie
- April Price
- Terry Spencer
- Shauna M Burke
- Eugenia Canas
- Rebecca J Haines-Saah
- Chantelle A Richmond
- Javeed Sukhera
Project Summary
Vaping (e-cigarette use) among youth in Canada has become a serious public health concern. Among teens in Canada (16-19 years), vaping prevalence increased from 29.3% in 2017 to 40.6% in 2019. The 2019 Canadian Tobacco and Drug Survey found that among youth aged 15-19, 87% had vaped nicotine and 40% had vaped cannabis in past 30 days. The history of failed tobacco cessation and substance use campaigns targeted at youth has shown that effective health promotion begins with evidence that centers young people’s experiences in meaningful ways.
Our “Teens Talk Vaping” project sought to co-produce research about teen vaping with teens to inform the development of evidence-based vaping education materials in collaboration with health authority and school board partners. Our participatory approach included a capacity-building programme to train teen team members as ‘co-researchers’, equipping them with the research skills necessary to contribute to all phases of the project. Paired with adult team members, teen co-researchers facilitated 7 online focus groups with teens (n=17) from across Canada, including teens who vaped and those who did not, to better understand how vaping features in their everyday environments. Using thematic analysis, our findings reveal the extent to which exposure to vaping is embedded in the everyday micro-geographies of Canadian teens across school environments, online spaces, and social settings.
To transform data into action, teen co-researchers engaged in creative workshops to develop creative products to disseminate key findings back to teens, including a short film (IHDCYH-Talks Special Commendation) and an Instagram campaign.
Project Updates: Short and/or long-term health effects of vaping (including lung injury in youth and/or adults)
-
Are e-cigarettes really a healthy alternative to combustible cigarettes? Cardiorespiratory, immune and thrombotic responses to smoking e-cigarettes vs combustible cigarettes under conditions of physical and mental stress
Nominated Principal Investigator
Simon Bacon
CIHR SPOR Chair in Innovative, Patient-Oriented, Behavioural Clinical Trials, Concordia University
simon.bacon@concordia.caPrincipal Investigator
Kim Lavoie
Knowledge User
Jean G Diodati
CIUSSS-NIMCo-investigators
- Jean Bourbeau
- Styliani Daskalopoulou
- Mathieu Morissette
- Nicola Paine
- Robert D Reid
Project Summary
Smoking is key to developing heart disease, lung disease, and cancer, with the number of people smoking highest in healthy young adults (20-24 years old). E-cigarettes (e-cigs) have been suggested as potential helping people stop smoking. However, we really don’t know how safe e-cigs are and the recent E-cigarette, or Vaping, product use-Associated Lung Injury (EVALI) events in the US and Canada have increased the need for more e-cig safety data.
Our study will look at how smoking e-cigs versus regular cigarettes versus nothing affects heart, blood, lung, and immune function during episodes of low intensity exercise and mental stress in young, otherwise healthy individuals who smoke. We will also test non-smokers (who won’t smoke but will do the exercise and mental stress task). Prior to the pandemic, we were able to recruit 24 participants (7 smokers and 17 non-smokers, 50% female).
This will be one of the first studies to assess the acute effects of e-cigs use (compared to regular cigarettes and doing nothing) influences stress induced physiological responses. Findings will generate new data that can be used to inform the safety debate about e-cig use, both in comparison to combustible cigarettes and to not smoking, that may be used to inform stakeholders (legislators, clinicians, patients) about their potential harms (or benefits) of ecigs, which may lead to appropriate regulation and use.
-
Lung health benefits of e-cigarette cessation
Nominated Principal Investigator
Christopher Carlsten
Professor, University of British Columbia
carlsten@mail.ubc.caPrincipal Investigator
Laura L Struik
Knowledge Users
- Christopher Lam
- Gerald B Thomas
Co-investigators
- Milan Khara
- Tina Afshar
Project Summary
The primary aim of the Lung Health Benefits of E-Cigarette Cessation study is to measure the airway and immune function of habitually vaping participants over 72 hours of quitting vaping.
Though initial participant recruitment began in early 2021, COVID-19 restrictions and amendments to the original study ethics application to include an additional breath sample collection and exhaled carbon monoxide measurement delayed the start of in-person visits.
Nonetheless, study recruitment has since progressed at a steady pace, with over 50 telephone screenings completed in the last year. The distribution of on-campus (UBC) advertisement material, combined with the resumption of in-person classes, and word of mouth between participants have been the main drivers of success in our recruitment recently. Recruitment efforts have also expanded to advertisements in local busses and REACH-BC.
The first in-person visit was conducted on January 4th, 2022. As of end March 2022, 13 individuals have completed their in-person clinical study visits and are in the progress of receiving virtual personalized biofeedback and follow-up. Three individuals have been scheduled for visits in the coming weeks, and an additional 18 eligible individuals are pending scheduling. Data obtained is processed as collected and stored for future analysis. We expect to reach our target of 30 participants by the end of summer 2022, in the absence of any major additional unforeseen delays.
-
VAPING: The unknown perils of inhalation and epithelial injury
Nominated Principal Investigator
Delbert Dorscheid
University of British Columbia
del.dorscheid@hli.ubc.caKnowledge User
Tawimas Shaipanich
University of British ColumbiaCo-investigators
- Tawimas Shaipanich
- Janice Leung
- Gurpreet Singhera
Project Summary
E-cigarette usage or "vaping" has increased in prevalence in recent years as an alternative to traditional cigarette smoking. 15% of Canadians have tried vaping and the majority of this group were youths and young adults aged 15-24. Liquid formulations that may or may not contain nicotine or tetrahydrocannabidiol, an active component of cannabis, are turned into vapour that is directly inhaled by the user. The wide variety of flavours available for vape liquids has made vaping especially appealing but little is known about its risks. In addition, a recent outbreak of vaping-related lung injury (EVALI) hospitalizations has led to growing concern regarding the dangers of this activity.
The lung is a network of branched tubes called airways and the cells which line these airways are important in maintaining lung health. These cells known as airway epithelial cells serve as an active barrier to inhaled air and the outside environment and thus are first point of contact for inhaled e-cigarette vapours. Irritants and other foreign particles often cause injury to this delicate layer of cells which lead to an inflammatory response and subsequent repair.
This project will focus on studying the effect of e-cigarette vapours on airway inflammation and how they may affect the lungs' innate repair mechanisms. We will measure key molecular markers of inflammation and molecular markers of injury to determine whether specific chemicals in e-liquids have a direct effect on the health of airway epithelial cells. Findings from this project will provide better understanding of the harmful effects of vaping on lung health. As vaping usage continues to increase among Canadians, it is critical that we utilize knowledge obtained from our study to better prevent and manage vaping related lung illnesses. By identifying the chemicals responsible for injury and understanding causes that lead to EVALI, we will be able to better treat and manage patients with vaping related lung illnesses.
-
Vaping Safety: A Knowledge Synthesis
Nominated Principal Investigator
Mark Eisenberg
Jewish General Hospital/McGill University
mark.eisenberg@mcgill.caKnowledge Users
- Trevor Mischki
(Principal Knowledge User)
Health Canada (Ottawa) - Hanan Abramovici
Co-investigators
- Andrea Benedetti
- Carolyn N Ells
- Kristian B Filion
- Andrea S Gershon
- Genevieve Gore
- Roland M Grad
- Brett D Thombs
Project Summary
Under this grant, we conducted a systematic review on the effects of e-cigarette use (or vaping) on lung function which has been submitted for publication. We screened 8856 abstracts and 44 full texts and identified 8 studies reporting on short and long-term effects of e-cigarette use on measures of lung function. These studies suggest that there are no acute effects of vaping on spirometry, but e-cigarette use may influence airway resistance and conductance. However, these results are suggestive rather than conclusive and further research is required. Our systematic review includes suggestions for future studies in this area, including recommendations to put more of a focus on long-term impacts of e-cigarette use.
When accepted, this systematic review will be published open-access to allow for dissemination of our findings. We plan to also share our findings by creating an InfoPOEM (Patient-Oriented Evidence that Matters), a 300-word synopsis of our research that could be delivered by the Canadian Medical Association (CMA) to its members, and by organizing a CIHR Café Scientifique which will be shared on YouTube and Facebook. This systematic review also contributed to the training of two new research assistants.
Our plans for this grant also include a systematic review on COVID-19 and vaping. This project aims to evaluate the incidence, severity, and long-term consequences of COVID-19 infection in those who vape compared to those who abstain from vaping.
- Trevor Mischki
-
Impact of cannabis vapor exposure on primary human airway epithelial cell immune responses
Nominated Principal Investigator
Jeremy Hirota
McMaster University
hirotaja@mcmaster.caPrincipal Investigator
James MacKillop
Knowledge User
James MacKillop
McMaster UniversityCo-investigators
- Andrew C Doxey
- Martin R Stampfli
Project Summary
Around the world there are an estimated 200 million cannabis users with the majority of these individuals using inhalation as the dominant delivery route. Canada is the first G8 country to legalize recreational cannabis use, with an estimated 20% of the population having consumed cannabis in the last year. Cannabis smoking exposes the breathing tubes in the lungs to combustion byproducts that have the potential to induce inflammation and swelling. The breathing tubes are lined with cells called "epithelial cells" and other immune cells that help fight infections. Our group has demonstrated that cannabis smoke exposure reduces the ability of some lung cells to fight infections. Cannabis vaping eliminates the combustion byproducts associated with cannabis smoking. Eliminating the cannabis byproducts may reduce the inflammation and swelling of the breathing tubes, although this remains to be determined. If demonstrated that vaping does not lead to inflammation and swelling of the breathing tubes, this delivery method could be an effective harm reduction strategy that could replace cannabis smoking.
We seek to determine how cannabis vaping exposure impacts human lung epithelial cell immune defences against viruses. Our project will use human lung epithelial cells from volunteer donors. We will grow these cells in plastic dishes that mimic the human lung environment. Using these human lung models, we will examine how exposure to increasing amounts of cannabis vapor impacts the ability of the cells to survive and defend against viruses that cause lung infections in humans. Our proposed studies will form a foundation for understanding the impact of cannabis vapor exposure on lung immune function in the context of clinically relevant viral exposures, guiding responsible use of cannabis by the population, regulation by government authorities, and production practices by commercial entities.
-
The pathogenesis of vaping-associated lung injury: Interactions between the alveolar epithelium and the immune system
Nominated Principal Investigator
Margaret Kelly
Professor, University of Calgary
mmkelly@ucalgary.ca
margaret.kelly@albertaprecisionlabs.caPrincipal Investigators
- Mark A Anselmo
- Matthias W Amrein
- Mark R Gillrie
- David Proud
Knowledge User
Mark Anselmo
Professor, University of CalgaryProject Summary
Vaping-associated lung illness (VALI) is a recently recognized lung injury related to the use of e-cigarettes. This study was designed to explore the underlying pathogenesis and is based on the hypothesis that disruption of surfactant by e-liquids generates acute inflammatory signals which damage the alveolar epithelial layer and further disrupt the surfactant layer.
Challenges due to the Covid-19 pandemic: All lab-based research at the University of Calgary was suspended from Mar - Sep 2020, after which labs could reopen only under special dispensation and without students. Some technologists were unable to return to work for a prolonged period of time as childcare was unavailable. In addition, several technologists have had to quarantine due to Covid infection/contacts. The delivery of the Multiplex Ion Beam Imager (MIBI), which will be used to image lungs with VALI, was delayed, and arrived January 2021. Optimization and training on the MIBI was challenging due to pandemic-related restrictions.
Experiments: A Vaping pen was used to deliver the e-liquid components PG:VG and nicotine, to culture media (‘e-sol’) which was then applied to bronchial epithelial cell cultures and to the Lung on a Chip model developed by Dr. Mark Gillrie. Primary human bronchial epithelial cells grown in an air-liquid interface housed in an exposure chamber have also been exposed directly to vape. Readouts of effects on cells includes morphology, cell viability, PCR, protein expression and Nanostring analysis. The effect of the e-liquid components and the e-sol on the integrity of surfactant have also been observed.
-
Utility of a preclinical model to study the impact of vaping products on cardiopulmonary outcomes
Nominated Principal Investigator
Koren Mann
McGill University
koren.mann@mcgill.caPrincipal Investigator
Carolyn Baglole
Knowledge User
Hanan Abramovici
Health CanadaCo-investigator
Jorg H Fritz
Project Summary
JUUL is a popular e-cigarette brand. Although there are a growing number of health concerns over vaping, we have very little information on the long-term consequences associated with use JUUL products. There is emerging evidence that e-cigarette aerosols induce inflammation that is linked to chronic lung and vascular diseases. However, these are conditions that take decades to develop in people, and thus epidemiological evidence that e-cigarette use contributes to these types of chronic diseases is not available.
To circumvent the need for epidemiological data on chronic health effects, which will take decades, we developed as part of our CIHR Catalyst grant, a preclinical mouse model to test the effects of vaping products on cardiopulmonary outcomes. We recently published a comparative study on popular JUUL flavors using an exposure scenario in mice that represents light/moderate exposure e-cigarette user (Been et al. Arch Toxicol. 2022). These data show that JUUL aerosols are not inert, and even an acute exposure induces pulmonary and systemic inflammatory and oxidative stress responses. We now have preliminary data that a 4 week “light” JUUL use further increases inflammation, and that there are important sex-specific differences in the lung “secretome”. Finally, using a mouse model of atherosclerosis, combined with our preclinical exposure model, we have evidence of early plaque development. While there is still a need for long-term epidemiological and clinical studies, this project is designed to provide rapid and relevant health information for vaping products currently available to Canadians on cardiopulmonary effects of vaping products.
-
Understanding the impact of vaping on innate immunity to respiratory viruses
Nominated Principal Investigator
Theo Moraes
Scientist, SickKids Research Institute
theo.moraes@sickkids.caPrincipal Investigator
Piushkumar Mandhane
Knowledge User
Piushkumar Mandhane
Univeristy of Alberta HospitalCo-investigator
Laurie A Zawertailo
Project Summary
We are interested in understanding if vaping can put someone at risk of having lung infections; and specifically, respiratory viral infections. To study this, we use models of lung epithelial cells that we grow in our lab and then expose these cells to aerosolized liquid from e-cigarettes. We can then infect these cells with respiratory viruses. Our initial work suggests there may be an impact of vaping on the amount of virus infection in lung epithelial cells. However, we also see that vaping alone leads to inflammation in epithelial cells. In the long run, this may be harmful to the lung. Our ongoing studies are focused on confirming these initial observations and on understanding the mechanisms that may link vaping to inflammation.
-
Novel pulmonary imaging of lung structure and function in symptomatic and asymptomatic e-cigarette smokers
Nominated Principal Investigator
Grace Parraga
University of Western Ontario
gparraga@uwo.ca
gparraga@robarts.caPrincipal Investigator
Constance A Mackenzie
Knowledge User
Constance Mackenzie
University of Western OntarioCo-investigators
- Karen J Bosma
- Inderdeep Dhaliwal
- Alexei Ouriadov
Project Summary
Modern electronic (e)-cigarettes were commercially developed in 2003 in China to provide an inhalable nicotine delivery device for current cigarette smokers in a safer, non-combustible manner with the long-term goal being harm reduction and enhanced combustible cigarette smoking cessation rates. While the impact of e-cigarettes on smoking cessation rates mainly stem from individual case reports, current vaping in otherwise never-smokers has been shown to be associated with 75% greater odds of developing lung diseases such as chronic bronchitis and emphysema, both of which are most commonly associated with combustible cigarette smoking. These startling results suggest that e-cigarette related pulmonary toxicity and damage occurs even in those who have never smoked combustible cigarettes. Importantly, the recent development of smaller, more portable, refillable and rechargeable e-cigarette devices in 2014 has led to a current explosion of e-cigarette use in teenagers and children who previously never smoked combustible cigarettes. In fact, as many as 1 in 4 Canadian teenagers used an e-cigarette in the past week, with sometimes deadly affects. Our team of investigators recently evaluated an 18-year-old e-cigarette user who survived life-threatening vaping-related airways disease and we continue to follow his recovery, 9 months post-ICU discharge. Here we aim to study how e-cigarette vapours and humectants impact the structure and function of the small airways and alveoli using MRI methods our team developed. We will also develop MRI biomarkers that simultaneously quantify respiratory and cardiovascular disease endpoints related to inflammation in a longitudinal cohort of e-cigarette users with direct comparison to combustible cigarette users and dual users.
-
Understanding the pathology of vaping associated lung damage in young adults
Nominated Principal Investigator
Christopher Pascoe
Assistant Professor, University of Manitoba
CPascoe@chrim.caKnowledge User
Neil Johnston
Manitoba Lung AssociationCo-investigators
- Neil Johnston
- Andrew J Halayko
- Biniam Kidane
- Paul Wawryko
Project Summary
Our study design uses lung tissue collected from lung surgery in young adults to compare changes in the overall transcriptome of the lung in e-cigarette users. Due to cancelled surgeries throughout the pandemic, we experienced significant delays in gathering this tissue. To date, we have 19 individuals recruited, 1 shy of our target. We are in the process of isolating RNA from the lung tissue for RNA-sequencing. In the interim, we have measured changes in plasma cytokines in a subset of samples and have performed patient call-backs as part of our retrospective analysis of archived tissue specimens from the previous two years. 2/3 of our e-cigarette users have never smoked cigarettes. 1/3 of them also smoke marijuana. Preliminary data in plasma suggest an increase in circulating pro-inflammatory cytokines in e-cigarette users.
-
Effects of ecigarettes on lung health: The VAPE Study (Vaping's airway and lung parenchymal effects)
Nominated Principal Investigator
Donald Sin
University of British Columbia
don.sin@hli.ubc.caKnowledge User
Menn Biagtan
BC Lung AssociationCo-investigators
- Menn Biagtan
- Rachel L Eddy
- Miranda A Kirby
- Jonathon Leipsic
- Janice Leung
Project Summary
The project launch was delayed owing to the start of the pandemic. We were able to initiate the project starting November, 2021 after the COVID-19 (research) restrictions at St. Paul’s Hospital and the University of British Columbia were lifted. Since then, we have received full institutional ethics board approval and began recruitment for both vaping and control subjects.
The control subjects consist of life-time non-smokers, ex-smokers and cannabis smokers. As of April 1, 2022, we have recruited 67 lifetime non-smokers and 71 active cannabis smokers (vaping and/or as cigarettes) and 3 vape-only smokers. All of these subjects will undergo extensive phenotyping with questionnaires, lung function measurements and imaging (thoracic CT scan and hyperpolarized xenon imaging). Approximately, 1/3 of the subjects will also undergo research bronchoscopies. We have completed the protocol on ~40 subjects and the others are in various stages of phenotyping.
To date, we have found that young smokers (whether vaping or cannabis) do not demonstrate any significant changes in lung function. However, some demonstrate abnormalities on thoracic CT scan (e.g. bronchiolitis) or xenon-129 MRI (e.g. ventilation defects). Our recruitment target for the vape-only group is 15 and we anticipate completion of recruitment by summer or fall of 2022.
-
Visualizing the effects of e-cigarette vape on alveolar macrophage function using a mouse model
Nominated Principal Investigator
Ajitha Thanabalasuriar
Assistant Professor, McGill University
ajitha.thanabalasuriar@mcgill.caPrincipal Investigator
Erika D Penz
Project Summary
Electronic cigarettes (e-cigs) were introduced as a tobacco smoking cessation product for adults. However, marketing tactics and the use of sweet and fruity flavours have resulted in e-cigs becoming increasingly popular among teenagers. Teens that have been using e-cigs for less than one year have been reported to develop rapid and life-threatening onset of severe lung injury and eosinophilic pneumonia. Vitamin E acetate in e-cigs has been linked to e-cig or vaping use associated-lung injury (EVALI) but the cause of eosinophilic pneumonia in e-cig users remains unclear. Lung injury is a consequence of increased infiltration and activation of the white blood cell such as neutrophils. Neutrophils and eosinophils are granulocytic innate immune cells that play major roles in pro- and anti-inflammatory (respectively) control of infections.
We have developed a mouse model of e-cig vape exposure using popular e-cig vape juice (berry mix) and e-cig devices (MOD) among teenage users. Using this mouse model, we have found that e-cig vape containing berry flavor impair neutrophil while increasing eosinophil migration to the lung. We are now using flow cytometry and RNA sequencing to understand the molecular mechanisms of impaired neutrophil and increased eosinophil trafficking to the lung. We hypothesize crosstalk between eosinophils and neutrophils after e-cig use can lead to improper activation of the cells and pneumonia or lung injury. Additionally, when we look at the different components of e-cig vape juice we see the presence of flavor has the most impactful effects on immune cell recruitment to the lungs.
-
Acute airway inflammation induced by vaping
Nominated Principal Investigator
Harissios Vliagoftis
University of Alberta
hari@ualberta.caPrincipal Investigator
Heather M Sharpe
Knowledge User
Heather M Sharpe
Canadian Network for Respiratory CareCo-investigators
- Paige Lacy
- Irvin Mayers
- Michael K Stickland
- Eric Y Wong
Project Summary
Our study aims to identify systemic and airway-specific acute and chronic inflammatory changes induced by vaping. For this we will study changes in activation status of inflammatory and immune cells and the presence of inflammatory mediators in blood and induced sputum before and after an acute vaping challenge. We will also compare baseline values between habitual vapers and naïve individuals to understand chronic inflammatory changes induced by vaping. We will recruit 20 habitual vapers and 20 subjects naïve to vaping and expose them to one acute session of vaping without nicotine.
We have developed flow cytometry protocols to consistently identify inflammatory cells (neutrophils, eosinophils, monocytes, macrophages and innate immune cells) in induced sputum and in peripheral blood. We have also developed assays to study superoxide production and phagocytosis by neutrophils and monocytes/macrophages.
The study has been delayed by COVID-19 restriction as for over a year we were unable to recruit subjects for this study and even now subjects are often reluctant to participate in studies. We have recruited and challenged 3 vaping naive subjects so far. Data from the 3 subjects we recruited indicate that the potential of peripheral blood monocytes and neutrophils to produce superoxide diminishes after a vaping session compared to a sham session and there is a trend for decreased phagocytosis under the same circumstances. Sputum data are under analysis.
Recruitment continues and we have more subjects scheduled for vaping challenges soon. -
Respiratory Effects of Nicotine and THC e-Cigarettes
Nominated Principal Investigator
Robert Schwartz
University of Toronto
Robert.Schwartz@utoronto.caPrincipal Investigators
- Peter Glazier
- Chung-Wai Chow
Knowledge User
Peter Glazier
Lung Health FoundationCo-investigators
- Michael O Chaiton
- Miranda A Kirby
- Micheal C McInnis
- Hui Peng
- Clodagh M Ryan
Project Summary
Both participant recruitment and clinical testing have been delayed, on and off, due to COVID-19. We began participant recruitment in Summer 2021 through social media and past study participant panels. To date, we have 15 participants enrolled in the clinical study (5 nicotine vapers; 10 non-vapers) out of 41 who were screened. We will continue to recruit participants until we reach our target sample of 36 vapers (12 exclusive nicotine, 12 exclusive THC, and 12 non-vapers).
Project Updates: Behaviours and associated health and social impacts of vaping product use (youth and/or adults)
-
Vaping and health outcomes, and use of the health care system among parents and adolescents in Manitoba
Nominated Principal Investigator
Tracie Afifi
Professor, University of Manitoba
tracie.afifi@umanitoba.caKnowledge User
Lil E Tonmyr
Public Health Agency of CanadaCo-investigators
- Lil E Tonmyr
- Marni D Brownell
- Harriet L Macmillan
- Nathan C Nickel
- Jitender Sareen
Project Summary
The current research will access the Well-Being and Experiences (The We Study) data, which includes a longitudinal adolescent (n = 1000) and parent/caregiver (n = 1000) community cohort from Manitoba. This research is novel because it will use longitudinal survey data from parents (baseline only) and adolescents (two waves) and linkages of these data to administrative health databases housed at the Manitoba Centre for Health Policy (MCHP) to further our understanding of vaping and health among adolescents and young adults.
The aims of the current research are as follows. 1) Does the course of vaping (i.e., never, cessation, new onset, or consistent) among adolescents and young adults and what factors (i.e., parental vaping and smoking cigarettes, adolescent sex [male or female], self-reported mental disorder, a history of child adversity, and a history of peer victimization) may be related to the course. This objective is complete and has been published in BMC Public Health. 2) Is vaping associated with poly substance use or new onset substance use over time (data analysis in progress). 3) Is vaping associated with increased odds of self-reported health conditions (data analysis in progress). 4) Is vaping used as a coping mechanism (data analysis in progress). 5) Does vaping have an impact on respiratory illness and use of the health care system (protocol for administrative data linkage complete.
Delays have been experienced due to the COVID-19 pandemic. However, all research objectives will be completed over the next 18 months.
-
A machine learning approach to identify drivers of e-cigarette dependence
Nominated Principal Investigator
Michael Chaiton
Independent Scientist, Centre for Addiction and Mental Health
michael.chaiton@utoronto.caKnowledge User
Peter Selby
Centre for Addiction and Mental HealthCo-investigators
- Susan J Bondy
- Adam G Cole
- Tara E Elton-Marshall
- Hayley A Hamilton
- Sean Hill
- Scott Leatherdale
- Nikolaos Mitsakakis
- Robert M Schwartz
- Wei Wang
Project Summary
Understanding person-level drivers of current e-cigarette use (vaping) is crucial to guide tobacco policy, but prior studies have not fully identified these drivers due to the reliance on cross-sectional data, small sample sizes in many studies, lack of generalizability, and limitations of traditional data analyses. This project has used machine learning techniques to examine predictors of vaping use and dependence among 5 separate population survey including three longitudinal cohorts.
Four papers have been published with 4 others in review or preparation. We additionally conducted a systematic review of machine learning studies in tobacco control (Fu et al., 2021). We used machine learning to examine intersectionality in the predictors of vaping dependence. Notably, Fu et al. found interactions were found between age and perceived discrimination, and between age and race/ethnicity, as those who were younger than their classmates and either reported experiencing discrimination frequently or identified as Asian or Native American/Pacific Islander were at increased risk of becoming frequent vapers. Shi et al (2022) found that 9 of the 10 top predictors of vaping had significant interactions with race. A longitudinal examination of the onset of dependence found that top predictors included cannabis use, purchasing vaping products at a grocery store, and use of food flavoured vaping liquid (Singh et al. In preparation).
Future work will include a pooled meta-analysis, methodology of examining intersectionality using machine learning, and examination of other health outcomes of vaping.
-
Genetic impact on youth vaping: Extending known genetic risk factors in smoking and tobacco-related illnesses to vaping
Nominated Principal Investigator
Meghan Chenoweth
Scientist, Centre for Addiction and Mental Health
meghan.chenoweth@utoronto.caPrincipal Investigator
Rachel F Tyndale
Knowledge Users
- Mark Eisenberg
McGill University - Amy Porath
Canadian Centre on Substance Use and Addiction
Co-investigators
- Mark J Eisenberg
- Amy J Porath
- David G Hammond
- Jennifer L O'Loughlin
- Marie-Pierre Sylvestre
Project Summary
Adolescents who smoke cigarettes are more likely to start vaping, and the reverse is also true: vaping can lead to smoking. While some young people report vaping to help them quit smoking, most continue to smoke resulting in dual use.
Genetic variation influences cigarette smoking. People with gene variants that increase the rate at which nicotine is inactivated smoke more cigarettes, have a higher risk for tobacco-related illnesses, and are less likely to quit smoking, compared to people with slow nicotine metabolism. In cohorts from Canada and England, we are studying whether youth smokers with genetically faster nicotine metabolism have a higher risk for becoming a dual user of cigarettes and e-cigarettes. In former smokers, we are also examining whether faster nicotine metabolism increases relapse back to smoking among vapers. While many young adults in Canada report vaping e-liquids that contain nicotine, e-liquids with cannabinoid extracts are also popular and we are investigating the effect of genetics on this choice. As a secondary goal, we are examining whether other genes, for example those that alter the response to nicotine and cannabis in the brain, also influence the risk for vaping.
The reasons underlying the surge in popularity of vaping among youth are not well understood, and our work will show whether genetic factors play a role. Studying youth from both Canada and England will help us to understand whether sociocultural or regulatory environments can influence this genetic risk.
- Mark Eisenberg
Project Updates: Mental health, addiction and dependence in the context of vaping (youth and/or adults)
-
Rewarding effects of "JUUL" e-cigarette vapour: Impact of age and neural correlates
Nominated Principal Investigator
Jibran Khokhar
Assistant Professor, University of Guelph
jkhokhar@uoguelph.caCo-investigator
Amy Estill
Project Summary
The aim of the following research is to assess developmental and sex differences in nicotine vapour-associated reward and withdrawal. We also explored the effects of sex and age on nicotine vapour pharmacokinetics and brain functional and structural connectivity.
Adult and adolescent rats of both sexes (n = 5-7/group) were exposed to either nicotine (JUUL, 5% nicotine) or vehicle vapour for 10 minutes and then assessed for either conditioned place preference (3 exposures paired with CPP chamber) or withdrawal (3x a day for 2 weeks). Rats were assessed for brain imaging 2 weeks after last of 14 days of exposure. Nicotine pharmacokinetics were assessed via blood draws after a single 10-minute exposure in a separate group of rats.
All groups (except adolescent females) showed significant increases in place preference for the nicotine-paired side, with adolescent males displaying significantly higher preference at lower doses than adult males. Moreover, only male adult and adolescent rats showing significant precipitated nicotine withdrawal. However, contrasting with these findings, female adolescent and adult rats had higher levels of nicotine and metabolites in the brain and plasma. Lastly, network-based statistics showed decreased functional and structural connectivity (across multiple nodes and edges) in rats exposed to nicotine (with sex and age as co-variates). Further analysis showed that there was a significant effect of sex on both structural and functional connectivity (specifically within corticostriatal circuitry).
Our results show that the reward- and withdrawal-like effects as well as physiological and pharmacological effects of nicotine vapour are age and sex dependent.
-
Health effects of vaping among youth: Evidence from quasi-experimental analyses
Nominated Principal Investigator
Van Hai Nguyen
Associate Professor, Memorial University of Newfoundland
hvnguyen@mun.caKnowledge User
David Diamond
Eastern HealthCo-investigators
- David S Diamond
- Stephen E Bornstein
- Leigh Anne Newhook
- Brenda J Wilson
Project Summary
Several studies have shown that vaping is associated with a number of adverse mental health outcomes. However, these studies are unable to establish the direction of this relationship. That is, it is not clear whether vaping causes adverse mental health, or people with mental health issues are more likely to vape.
In this study, we first estimated the impacts of minimum legal age (MLA) laws for e-cigarettes on youths' mental health (mood and anxiety disorders) and their e-cigarette use. Next, we combined these estimates to generate the causal effect of vaping on mental health. We used data from the nationally representative Canadian Community Health Surveys 2008-2019 and Canadian Student Tobacco Alcohol and Drugs Surveys 2008-2019.
We found that MLA laws for vaping reduced the risks of mood and anxiety disorders. These reductions are likely driven by lower cannabis and illicit drug use, and improved peer relationships at schools. Combined with previous evidence that the MLA law also reduced youth e-cigarette use, our findings suggest that youth e-cigarette use leads to higher risks of mood and anxiety disorders.
Our study draws attention to e-cigarettes as a contributor to the growing mental health crisis facing youths and heightens the need to address rising youth e-cigarette use. It also highlights the MLA law's benefits of reducing these risks by lowering youths' cannabis and illicit drug use and enhancing their feeling of being part of schools.
-
Vaping in at-risk populations: Effects on mental and physical health (VAPE) study
Nominated Principal Investigator
Zainab Samaan
Professor, McMaster University
samaanz@mcmaster.caPrincipal Investigator
Leonora J Regenstreif
Knowledge Users
- Leonora J Regenstreif
McMaster University - Tea Rosic
University of Ottawa
Co-investigators
- Tea Rosic
- Claire de Oliveira
- Alessia D'Elia
- David C Marsh
- Parameswaran K Nair
- Nitika Sanger
- Lehana Thabane
Project Summary
Vaping is prevalent within the opioid use disorder (OUD) population, with data suggesting 19.2% of OUD patients reporting vaping of substances including nicotine, cannabis and flavoured waters. Given the prevalence of vaping, and the co-occurrence of mental health challenges and polysubstance use with vaping, it is critical to understand perceptions and motivations for vaping within individuals with OUD and the impact of vaping on opioid use, health conditions and treatment outcomes in this high risk population.
Using different approaches to address the study questions, including observational, qualitative and data linkage methods, we provide the findings to date. The qualitative study included patients with OUD, aged 36.47 (SD=6.42)) years, and 53.8% of participants were female. Participants were predominantly European ancestry (84.6%), cisgender (100%), single (69.2%), and currently receiving methadone for OUD treatment (84.6%). The mean age when first introduced to vaping and first began vaping regularly was 30.23 (SD=8.26) and 32.08 (SD=7.19), respectively. Majority (92%) reported daily vaping. The common substances vaped were nicotine (53.8%), flavoured nicotine (38.5%), THC (15.4%) and CBD (7.7%). Motivation for vaping included "get high, reduce cravings, boredom, others using, relief stress and anxiety" The observational study component included 2247 patients with OUD, 461 individuals reported vaping. Individuals reported vaping were more likely to report chronic pain (22% vs 18%), current cannabis use (61% vs 48%), no difference in history of opioid overdose (32%) by vaping status. Ongoing opioid use during treatment was seen in 15% of those who vape versus 12% of those who don't vape.
- Leonora J Regenstreif
Project Updates: Vaping policy issues related to youth and/or adults
-
An experimental investigation of the demand for electronic nicotine delivery systems (ENDS)
Nominated Principal Investigator
Emmanuel Guindon
Associate Professor, McMaster University
emmanuel.guindon@mcmaster.caPrincipal Investigators
- Neil J Buckley
- Emmanouil Mentzakis
Knowledge Users
- Evan Blecher
Institut national de santé publique du Québec - Annie Montreuil
World Health Organization
Co-investigators
- Michael O Chaiton
- Paul Rodriguez-Lesmes
- Ce Shang
- Arthur Sweetman
- Cynthia Callard
- Les Hagan
Project Summary
Our project aimed to examine, using experimental approaches, the demand for electronic nicotine delivery systems (ENDS) such as vaping and heated products. Few important ENDS policy changes had been implemented in Canada and for the relatively few that had been implemented, changes occurred recently which makes it difficult to use standard empirical approaches.
Specifically, we examined the effects of, and trade-offs between: 1) health warning on devices and combustible cigarettes; 2) plain packaging of vaping products; 3) maximum nicotine level allowable in vaping products, and, 4) prices. Although our focus was on the demand for popular vaping and heated products, we included combustible cigarettes as an alternative in our study design because of the importance of interactions between ENDS and combustible cigarettes. To keep our experiment as realistic as possible, we focused on the most popular brands for each product category (JUUL, vaping; IQOS, heated; and du Maurier, cigarettes).
We examined ENDS use intentions and risk perceptions among approximately 1800 young nonusers (16 to 29 years) residing in Alberta, Ontario, and Québec. Preliminary analyses indicates that:
- Young nonusers were more likely to choose vaping or heated products when asked which option would encourage someone like them to try.
- Higher prices of any products increased the use intentions of alternative products; this was particularly salient for combustible cigarettes (i.e., higher prices for vaping and heated products increased cigarette use intentions).
- Lower nicotine strength of vaping product increased use intentions.
Plain packaging and higher nicotine strength of vaping product increased risk perception.
-
The impact of vaping policies on the e-cigarette product market and youth vaping
Nominated Principal Investigator
David Hammond
University of Waterloo
dhammond@uwaterloo.caKnowledge User
Manuel Arango
Heart and Stroke FoundationCo-investigators
- Katherine East
- Christian Boudreau
- Geoffrey T Fong
- Richard J O'Connor
Project Summary
In May 2018, e-cigarettes containing nicotine became legally available for sale in Canada under the TVPA, along with increased marketing and retail access for major international brands, such as JUUL. In the following two years, the prevalence of youth vaping in Canada doubled. In response, provinces have announced a range of policy measures to be implemented in 2020—including restrictions on retail access, flavours, nicotine, and product design. Policy variation across provinces provides a unique opportunity for a 'natural experiment' research design.
The proposed study has two primary objectives: to examine the impact of provincial policies on: 1) e-cigarette retail availability, and 2) vaping behaviour among young people. The study consists of two data sources. First, an environmental scan of the e-cigarette market will be conducted in each province to collect information about the retail availability, nicotine content, and flavours of e-liquid products on the market. Second, data from the environmental scan will be linked with data from the ITC Youth Tobacco and Vaping Surveys, which consists of population-based surveys of youth aged 16-19 in Canada, conducted annually between 2017 and 2021.
Analyses will examine pre-post changes within provinces for four primary outcomes: 1) retail access and purchase source of e-cigarettes; 2) use of flavoured e-cigarettes; 3) product type, including nicotine concentration and use of salt-based e-cigarettes; and 4) overall changes in the prevalence of vaping across provinces. Findings will be used to show how the industry has responded to policies and the impact on youth vaping.
-
Geospatial analyses of vape retailer accessibility: Examining socioeconomic and environmental determinants
Nominated Principal Investigator
Jamie Seabrook
Brescia University College
jseabro2@uwo.caPrincipal Investigator
Jason A Gilliland
Co-investigators
- Christopher Mackie
- Kelly K Anderson
- Gina Martin
- Jacob J Shelley
Project Summary
There is an urgent need to develop tools and measures to understand predictors of youth vaping and to guide policy and programs aimed at reducing harms from youth vaping. With the changing regulatory landscape concerning e-cigarette sales and advertising across Canada, it is imperative that we monitor the effects of vape availability from retail access.
We currently know little about the association between environmental factors (availability and visibility of vape products) and youth vaping. Although legally one must be of majority age to purchase vaping devices and products, there has been limited regulatory enforcement from retail outlets, allowing teenagers easy access.
Despite pandemic-related setbacks, our team has managed to create a database for all Ontario that identifies and maps the locations of every vape retailer utilizing geospatial analysis techniques within a geographic information system. We created metrics such as "proximity to" and "density of" vaping retailers in relation to school locations and neighbourhood socioeconomic status across the province and identified socioeconomic inequities regarding the distribution of this environmental burden.
Upon completion of the project, a database of outcome measures will be made freely available to other researchers and public health professionals. This open dataset will support the linking other datasets to conduct further research into the determinants of youth vaping and related health outcomes, as well as the impacts of future regulatory changes. We hope that the outcomes of this research project will help inform the development of future prevention and awareness campaigns, as well as regulations restricting youth vaping.
Project Updates: Other areas related to the health consequences of vaping in youth and/or adults
-
Cannabis and nicotine vaping during pregnancy and postpartum
Nominated Principal Investigator
Lorraine Greaves
Senior Investigator, Centre of Excellence for Women's Health
lgreaves@cw.bc.caPrincipal Investigator
Nancy Poole
Knowledge User
Jocelynn L. Cook
Chief Scientific Officer, Society of Obstetricians and Gynaecologists of CanadaCo-investigator
Jocelynn L Cook
Project Summary
Little is known about vaping cannabis and/or nicotine during pregnancy and postpartum. We conducted 111 surveys and 22 semi-structured interviews in 2020-2021 with women who vape(d) nicotine and/or cannabis during pregnancy/postpartum reporting their experiences, motivations and information seeking. Recruitment was done using social media (Instagram, Facebook, and Twitter) and websites and social media pages of relevant external organizations.
Survey data were analyzed using SPSS and interview transcripts using NVivo. 63% of respondents were currently pregnant, 51% vaped nicotine, 27.9% vaped cannabis, and 20.7% vaped nicotine and cannabis. 68.5% vaped daily. The most frequent reasons for vaping cannabis were to manage insomnia, depression and anxiety, headaches/migraines, and lack of appetite, and to avoid pharmacological alternatives perceived as more harmful. Vaping nicotine was primarily undertaken to reduce or quit smoking. For women who consulted health care providers about vaping either cannabis or nicotine, the most frequent reasons were to understand possible harms during pregnancy, and to fetal or child health. Interview data highlighted: women´s agency in seeking information and decision-making; processes of assessing risks and balancing benefits and harms; the reasons for vaping; context of use; and experiences of internal and external stigma. Women described the influences on their processes, such as health care providers, friends, partners, online support groups, and online information. Participants made vaping related decisions after considerable input and were generally critical assessors of information.
Findings will be translated into information sheets, conversation starters, video and tweetorials for healthcare providers, a journal article and conference presentations.
-
Vaping and asthma - A study on short and long-term health effects of vaping among Ontario youths and young adults with asthma identified in health administrative data and linked to the Canadian community health survey
Nominated Principal Investigator
Teresa To
PhD Senior Scientist, The Hospital for Sick Children
teresa.to@sickkids.caKnowledge User
Anne Van Dam
Canadian Thoracic SocietyCo-investigators
- Jennifer MacKinnon
- Cornelia M Borkhoff
- Chung-Wai Chow
- Theo Moraes
- Robert M Schwartz
- Nicholas Vozoris
Project Summary
Background and Methods: Recent youth hospitalizations suggests that e-cigarette (EC) use may have long-term health effects. This cohort study used linked Canadian Community Health Survey (CCHS) and health administrative data from January 1, 2015-March 31, 2018 to determine whether EC users and non-users aged 15-30 years had differing odds of asthma, asthma attacks and patterns of health services use (HSU). This study consisted of 2,700 CCHS participants. Matched multivariable logistic regression was used to calculated odds ratios (OR) and 95% confidence intervals (CI) with asthma and asthma attacks. Multivariable negative binomial regression was used to estimate rate ratios (RR) with 95% CI of all-cause HSU (hospitalization, emergency department visit).
Findings: After adjusting for confounders, EC users had 21% higher odds of having asthma (OR=1.21; 95% CI: 0.95-1.54). Among those with asthma, EC users had greater than two-fold higher odds of having an asthma attack in the last 12 months (OR=2.30; 95% CI: 1.29-4.12). When stratified by sex, female cigarette and EC smokers and non-EC smokers had significantly increased all-cause HSU (RR=1.93; 95%CI:1.39-2.68 and RR=1.41; 95%CI: 1.16-1.71, respectively). However, EC use was not significantly associated with all-cause HSU among the asthma population nor with respiratory-disease-specific HSU.
Conclusion: In conclusion, Current EC use is associated with significantly increased odds of having asthma and asthma attacks. Furthermore, concurrent EC use and cigarette smoking are associated with a higher rate of all-cause HSU and was the highest among women. Our findings suggest that EC use may be an epidemiological biomarker for youth and young adults with increased health morbidity.
Appendix A – Whiteboard: Research Related Challenges and Mitigation Strategies
Research Related Challenges and Mitigation - Board 1
Moderator
Michael Chaiton
Facilitator
Lauren Tierney
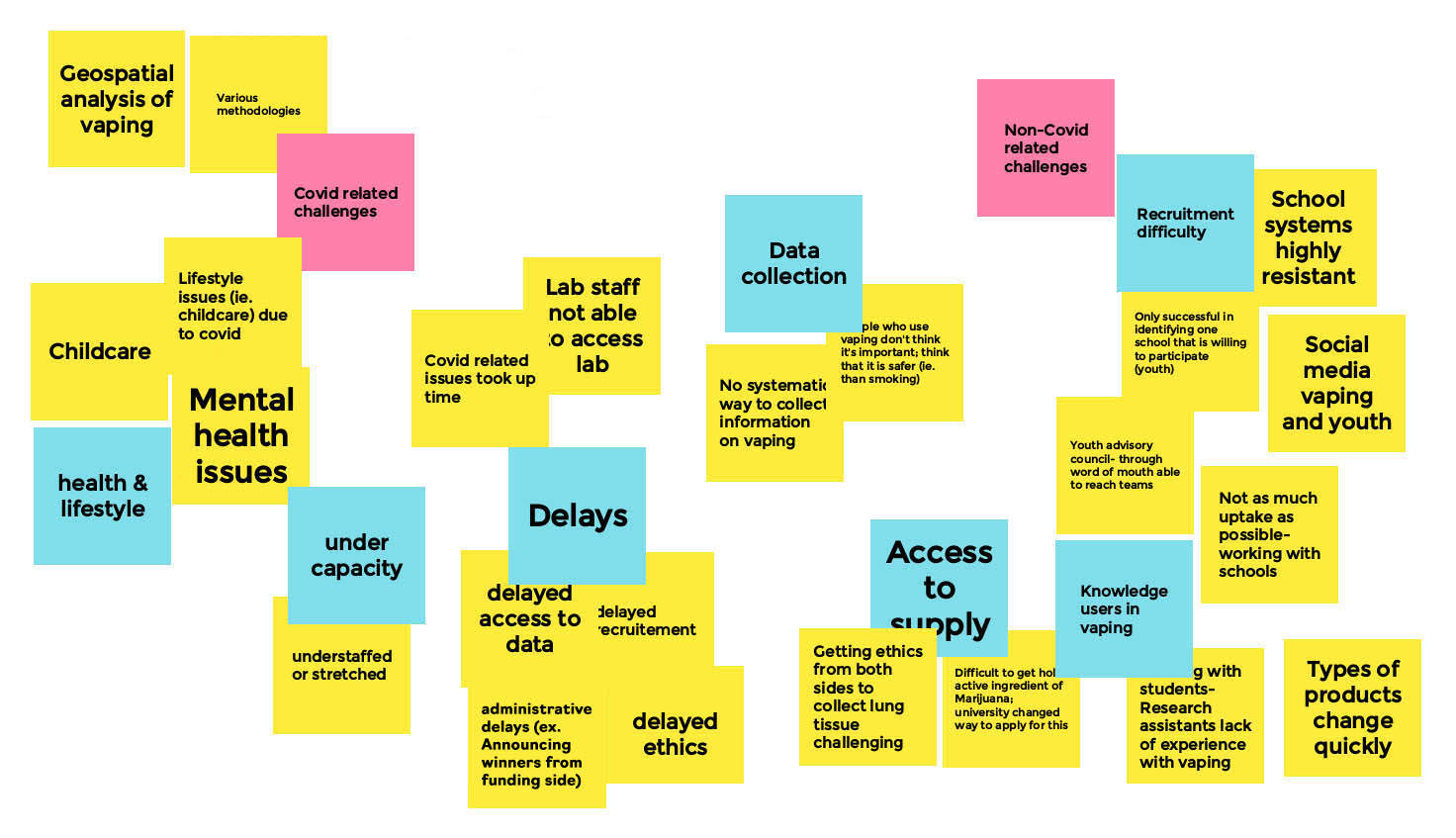
Long Description
- COVID Related Challenges
- Health & Lifestyle
- Childcare
- Mental health issues
- Lifestyle issues (i.e. Childcare) due to covid
- Geospatial analysis of vaping
- Various methodologies
- Under Capacity
- Understaffed or stretched
- Delays
- Covid related issues took up time
- Lab staff not able to access lab
- Delayed access to data
- Delayed recruitment
- Administrative delates (ex. Announcing winners from funding side)
- Delayed ethics
- Health & Lifestyle
- Non-Covid related Challenges
- Data Collection
- No systematic way to collect information on vaping
- People who use vaping don't think it's important; think that it is safer (i.e. Than smoking)
- Access to Supply
- Getting ethics from both sides to collect lung tissue challenging
- Difficult to get ahold of active ingredients of Marijuana; university changed way to apply for this
- Types of product change quickly
- Knowledge users in vaping
- Working with students/research assistants lack of experience with vaping
- Recruitment difficulty
- School systems highly resistant
- Only successful in identifying one school that is willing to participate (youth)
- Social media, vaping, and youth
- Youth advisory council through word of mouth, are able to reach teams
- Not as much uptake as possible - working with schools
- Data Collection
Research Related Challenges and Mitigation - Board 2
Moderator
Jibran Khokhar
Facilitator
Sara Kelly
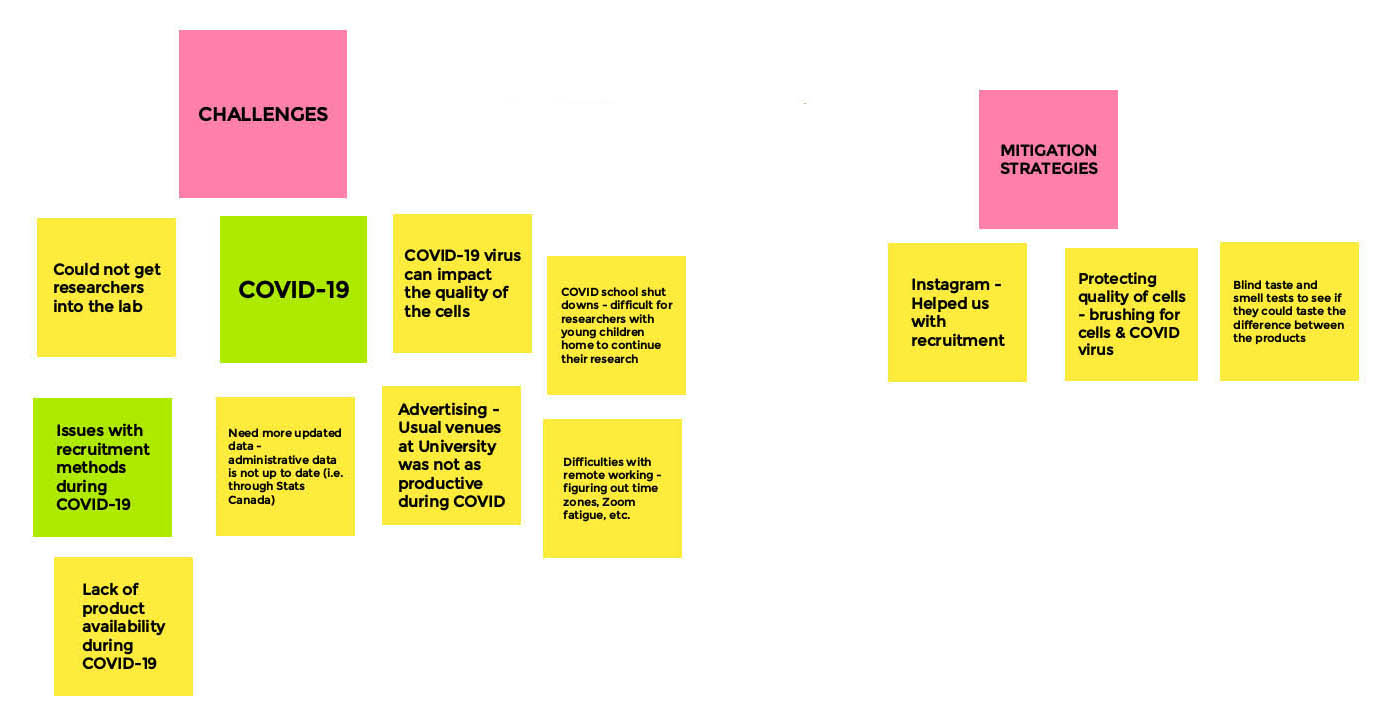
Long Description
- Challenges
- COVID-19
- Could not get researchers into the lab
- COVID-19 virus can impact the quality of cells
- COVID school shutdowns - difficult for researchers with young children home to continue their research
- Advertising — usual venues at university was not as productive during COVID
- Difficulties with remote working - figuring out time zones, Zoom fatigue, etc.
- Issues with recruitment methods during COVID-19
- Lack of product availability during COVID-19
- Need more updated data - administrative data is not up to date (i.e. through stats Canada)
- COVID-19
- Mitigation Strategies
- Instagram helped us with recruitment
- Protecting quality of cells - brushing for cells & COVID virus
- Blind taste and smell tests to see if they could taste the difference between the products
Research Related Challenges and Mitigation - Board 3
Moderator
Donald Sin
Facilitator
Shannon Wowk
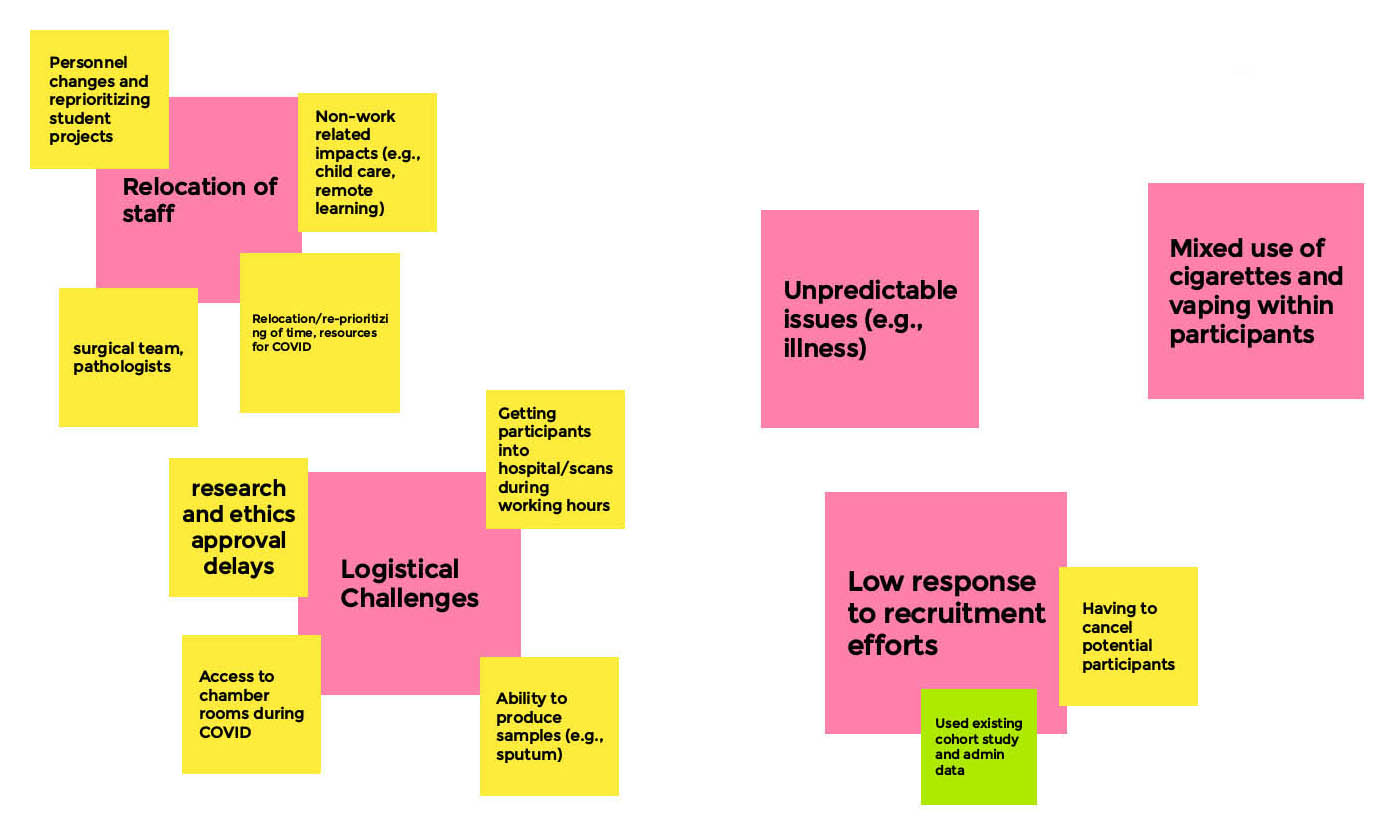
Long Description
- Relocation of Staff
- Personnel changes and reprioritizing student projects
- Surgical team, pathologists
- Non-work related impacts (e.g. child care, remote learning)
- Relocation/re-prioritizing of time, resources for COVID
- Logistical Challenges
- Research and ethics approval delays
- Getting participants into hospital/scans during working hours
- Access to chamber rooms during COVID
- Ability to produce samples (e.g. sputum)
- Unpredictable issues
- E.g. illness
- Low response to recruitment efforts
- Having to cancel potential participants
- Used existing cohort study and admin data
- Mixed Use of cigarettes and vaping within participants
Research Related Challenges and Mitigation - Board 4
Moderator
Ajitha Thanabalasuriar
Facilitator
Joanne Wincentak
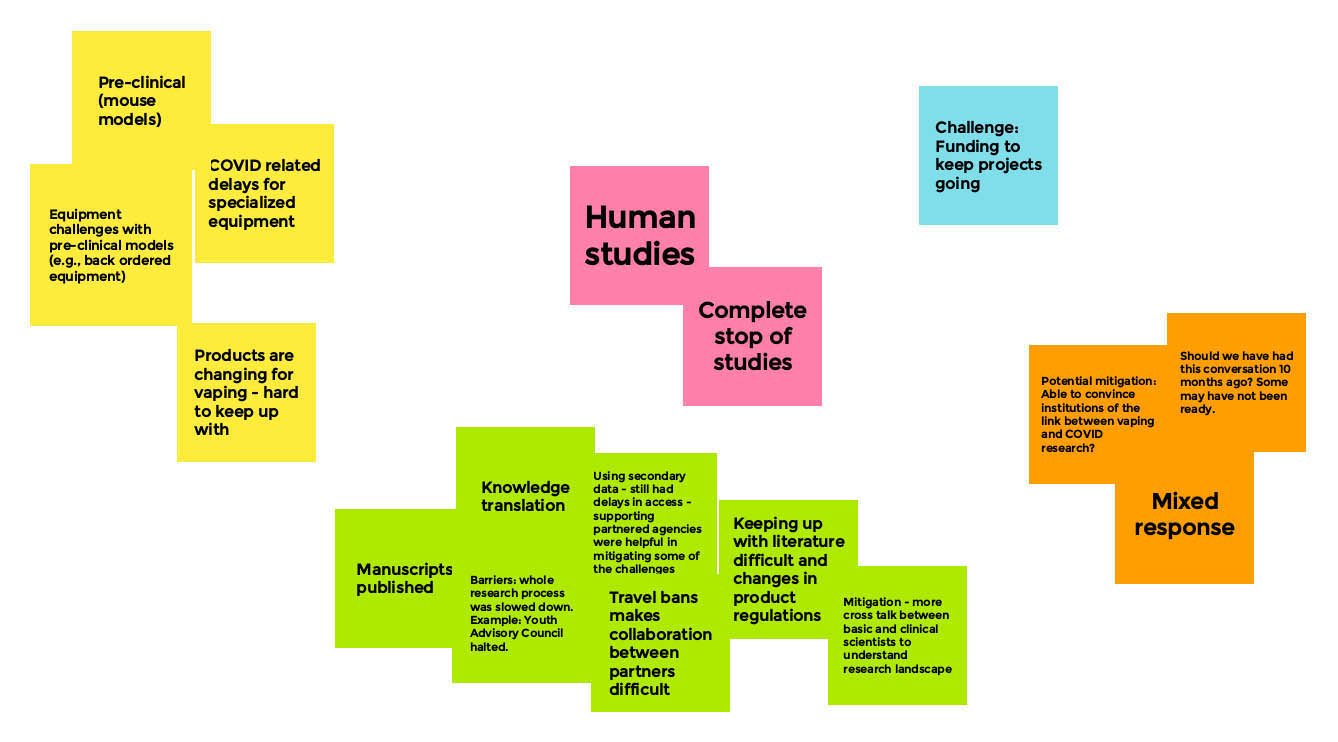
Long Description
- Pre-clinical (mouse models)
- COVID related delays for specialized equipment
- Equipment challenges with pre-clinical models (e.g. back order equipment)
- Products are changing for vaping - hard to keep up with
- Human studies
- Complete stop of studies
- Challenge : funding to keep projects going
- Manuscripts published
- Knowledge translation
- Barriers: Whole research process was slowed down. Example: Youth advisory council halted
- Using secondary data — still had delays in access — supporting partnered agencies were helpful in mitigating some of the challenges
- Travel bands makes collaboration between partners difficult
- Keeping up with literature difficult and changes in product regulations
- Mitigation — more cross talk between basic and clinical scientists to understand research landscape
- Potential mitigation: able to convince institutions of the link between vaping and COVID research?
- Should we have had this conversation 10 months ago? Some may have not been ready
- Mixed response
Appendix B – Whiteboard: Next Steps and Opportunities for Collaboration
Next Steps and Opportunities for Collaboration - Board 1
Moderator
Michael Chaiton
Facilitator
Lauren Tierney
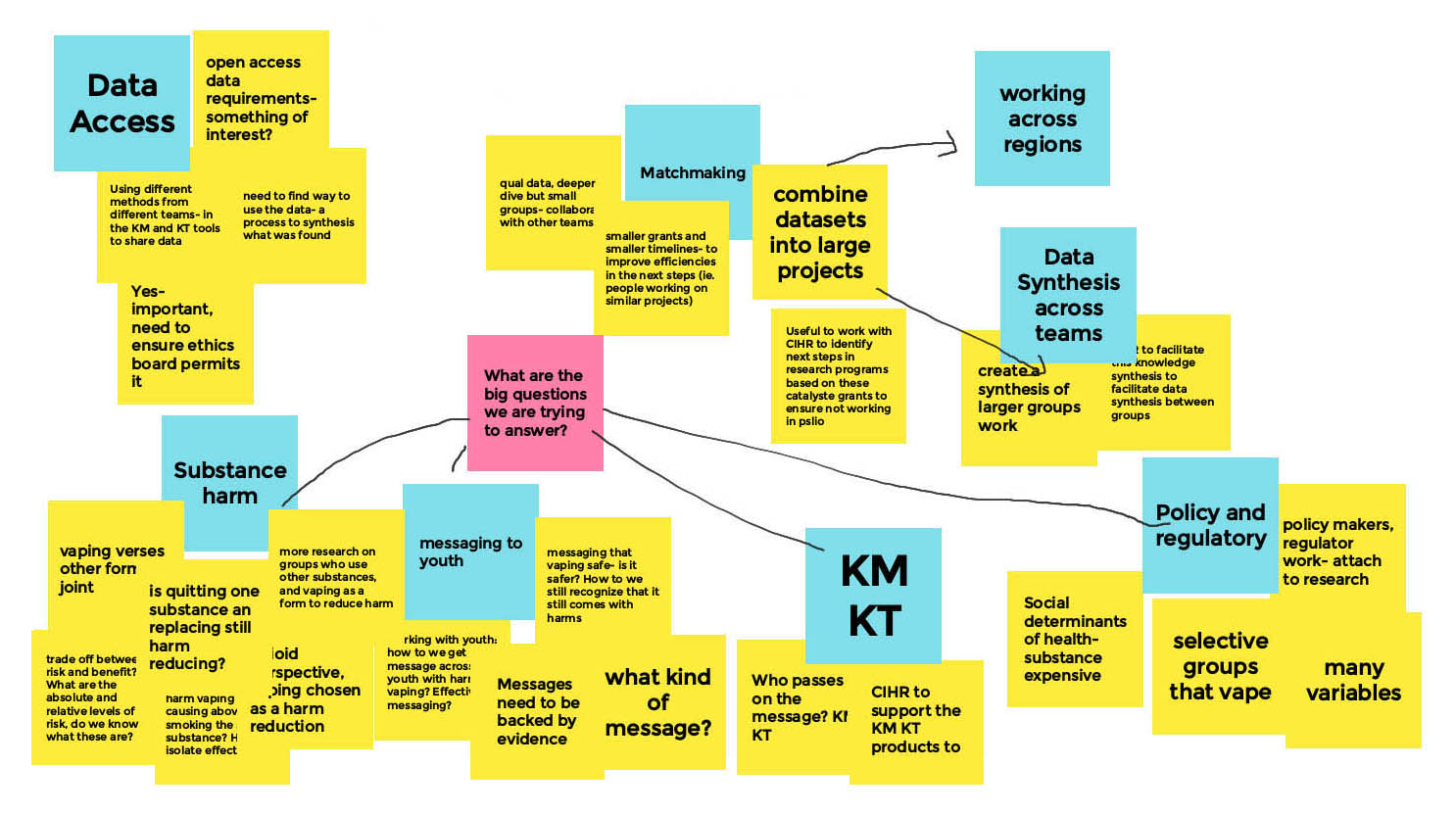
Long Description
- Data Access
- Open access data requirements- something of interest?
- Using different methods from different teams - in the KM and KT tools to share data
- Yes important, need to ensure ethics board permits it
- Need to find way to use the data - a process to synthesis what was found
- What are the big questions we are trying to answer?
- Substance Harm
- Vaping versus other form, ex. joint
- Tradeoff between risk and benefit? What are the absolute and relative levels of risk, do we know what these are?
- Is quitting one substance and replacing still harm reducing?
- Vaping and nicotine - Is the harm vaping causing above smoking the same substance? How to isolate effect
- More research on groups who use other substances and vaping as a form to reduce harm
- From an opioid perspective, vaping chosen as a harm reduction
- Messaging to Youth
- Working with youth, how do we get message across to youth with harms of vaping? Effective messaging?
- Messaging that vaping is safe - is it safer? How do we still recognize that it still comes with harms
- Messages need to be backed by evidence
- What kind of message?
- Knowledge Mobilization / Knowledge Translation (KM/KT)
- Who passes on the message? KM/KT
- CIRH to support the KM KT products to come
- Substance Harm
- Policy and Regulatory
- Social determinants of health - substance expensive
- Selective groups that vape
- Many variables
- Policy makers, regulatory work, attach to research
- Matchmaking
- Qualitative data, deeper dive but smaller groups — collaborate with other teams
- Smaller grants and smaller timelines - to improve efficiencies in the next steps (i.e. People working on similar projects)
- Combine datasets into large projects
- Useful to work with CIHR to identify next steps in research programs based on these catalyst grants to ensure not working in psilo
- Data Synthesis across teams
- Create a synthesis of larger groups work
- CIRH to facilitate this knowledge synthesis to facilitate data synthesis between groups
- Working across regions
Next Steps and Opportunities for Collaboration - Board 2
Moderator
Jibran Khokhar
Facilitator
Sara Kelly
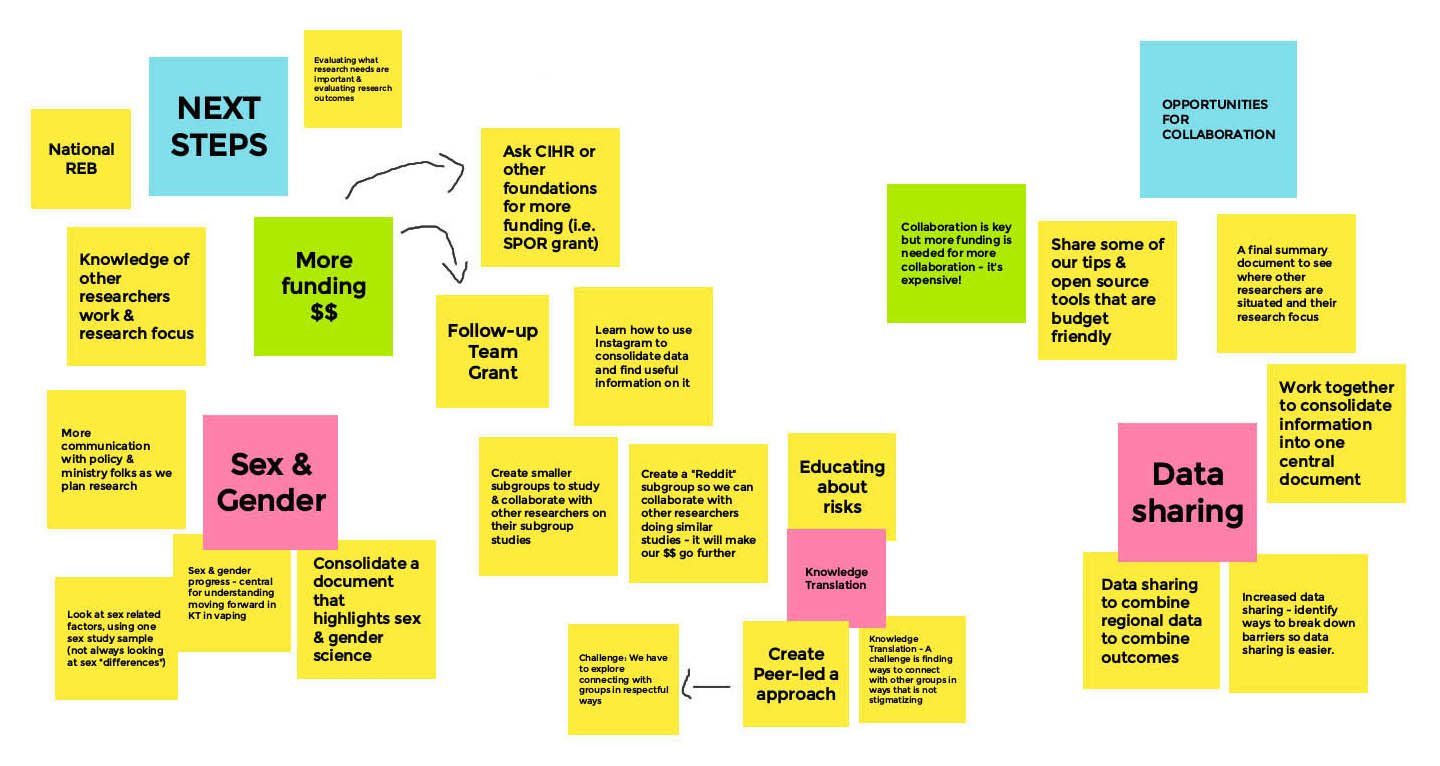
Long Description
- Next Steps
- More Funding
- Ask CIHR or other foundations for more funding (i.e. SPOR grant)
- Follow up Team Grant
- Sex & Gender
- Look at sex related factors, using one sex study sample (not always looking at sex "differences")
- Sex & gender progress- central for understanding moving forward in KT in vaping
- Consolidate a document that highlights sex & gender science
- Knowledge Translation
- Knowledge translation — a challenge is finding ways to connect with other groups in ways that is not stigmatizing
- Create peer-led approach
- Challenge : we have to explore connecting with groups in respectful ways
- Educating about risks
- More communication with policy & ministry folks as we plan research
- Opportunities for Collaboration
- Create smaller subgroups to study & collaborate with other researcher on their subgroup studies
- Create a "reddit" subgroup so we can collaborate with other researchers doing similar studies - it will make our money go further
- Knowledge of other researchers work & research focus
- Collaboration is key but more funding is needed for more collaboration - it's expensive!
- Share some of our tips & open-source tools that are budget friendly
- A final summary document to see where other researcher are situated with their research focus
- National REB
- Evaluating what research needs are important & evaluating research outcomes
- Data Sharing
- Work together to consolidate information into one central document
- Data sharing to combine regional data to combine outcomes
- Increased data sharing – identify ways to break down barriers so data sharing is easier
- Learn how to use Instagram to consolidate data and find useful information in it
Next Steps and Opportunities for Collaboration - Board 3
Moderator
Donald Sin
Facilitator
Shannon Wowk
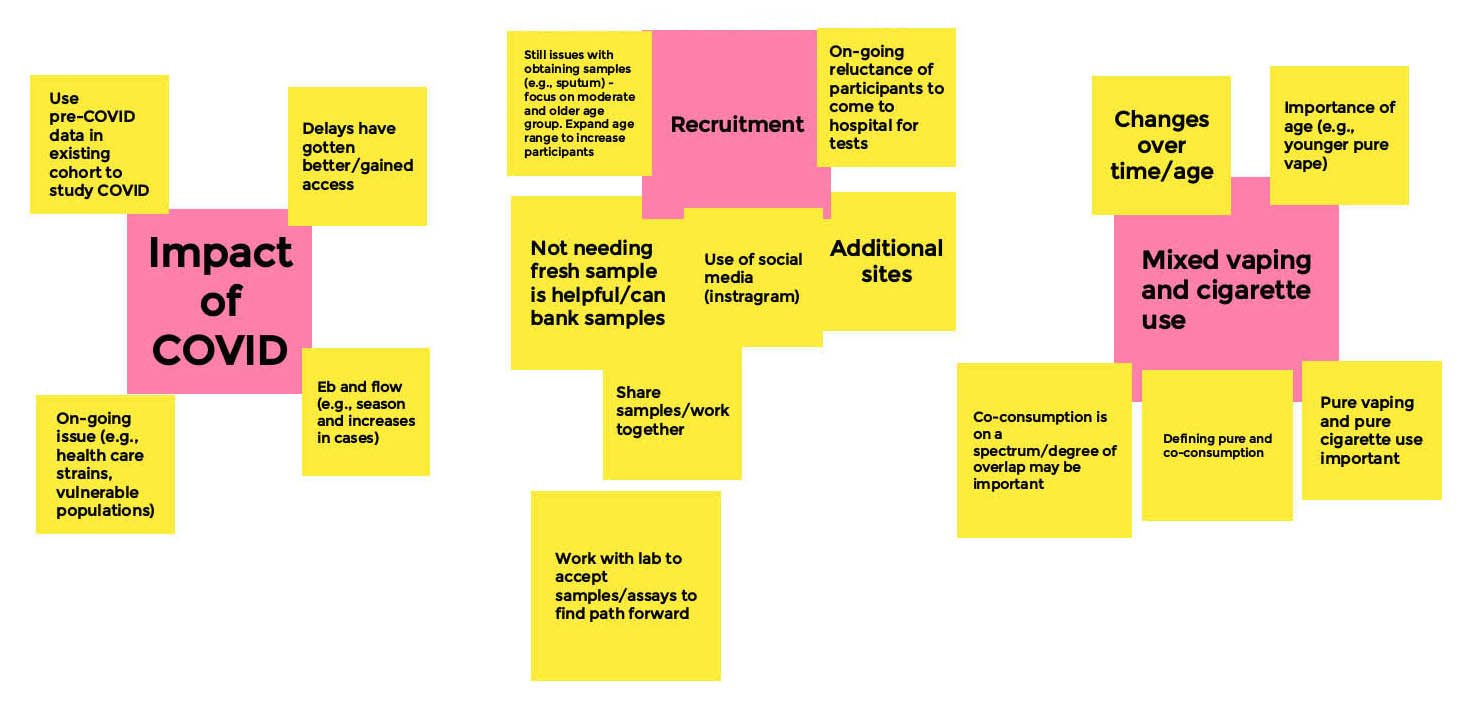
Long Description
- Impact of COVID
- Use pre-COVID data in existing cohort to study COVID
- Delays have gotten better/ gained access
- On-going issues (e.g. health care strains, vulnerable populations)
- Eb and flow (e.g., season and increases in cases)
- Recruitment
- On-going reluctance of participants to come to hospital for tests
- Additional sites
- Use of social media (Instagram)
- Share samples/work together
- Not needing fresh sample is helpful/can bank samples
- Share samples/work together
- Work with lab to accept samples/assays to find path forward
- Mixed Vaping and Cigarette Use
- Changes over time/age
- Importance of age (e.g., younger pure vape)
- Co-consumption is on a spectrum / degree of overlap may be important
- Defining pure and co-consumption
- Pure vaping and pure cigarette use important
Next Steps and Opportunities for Collaboration - Board 4
Moderator
Ajitha Thanabalasuriar
Facilitator
Joanne Wincentak
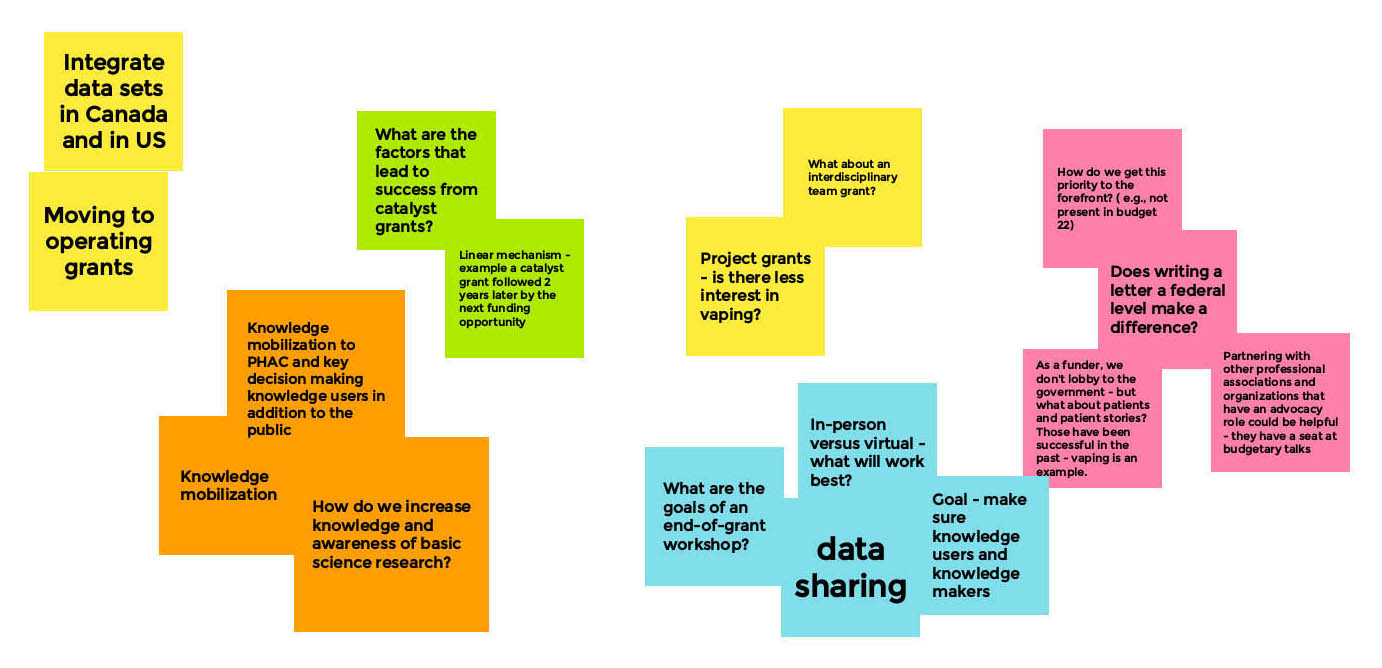
Long Description
- Integrate data sets in Canada and in US
- Moving to operating grants
- What are the factors that lead to success from catalyst grants?
- Linear mechanism — e.x. a catalyst grant followed 2 years later by the next funding opportunity
- Knowledge mobilization to PHAC and key decision - making knowledge users in addition to the public
- Knowledge Mobilization
- How do we increase knowledge and awareness of basic science research?
- What about an interdisciplinary team grant?
- Project grants - is there less interest in vaping?
- Data sharing
- What are the goals of an end of grant workshop?
- In-person versus virtual what will work best?
- Goal — make sure knowledge users and knowledge makers
- How do we get this priority to the forefront? (e.g., not present in budget 2022)
- Does writing a letter at the federal level make a difference?
- As a funder, we don't lobby to the government - but what about patients and patient stories? Those have been successful in the past - vaping is an example
- Partnering with other professional associations and organizations that have advocacy role could be helpful - they have a seat at the budgetary talks
- Date modified: