Meet the Methods series: Measuring and Manipulating Sex Hormones in Laboratory Animals
Issue 1 - August 2020

In basic science, sex hormones can be measured and manipulated in mice and rats using a variety of techniques. The CIHR Institute of Gender and Health asked Margaret McCarthy, Ph.D., Professor of Pharmacology at the University of Maryland, about her views on how to best integrate these methods in her research. Margaret McCarthy’s research focuses on the influence of sex hormones on the developing brain, with a special emphasis on understanding the cellular mechanisms that establish sex differences. Here are Margaret McCarthy’s recommendations:
When should studies with female mice or rats be staging the estrous cycle?
Start by overlooking the estrous cycle. Look at your data and see if there is variability in your male or female experimental groups. Variability in males may be due to group housing, which can alter steroid levels in mice and rats. Learn moreFootnote 1.
If there is low variability in males, but high variability in females, then it is advisable to stage the estrous cycle to see if this is a source of variability. To do this, determine and monitor the stage of the estrous cycle by visual observation or vaginal cytology over 2 weeks to uncover how changes in estrogen and progesterone levels may be important for your experimental endpoints. Learn moreFootnote 2.
TIP: Estrous cycle dynamics can vary between different strains of mice and rats.
What assays do you recommend to quantify sex hormone levels in mice and rats?
There are several assays that can be used to measure androgens, estrogens and progesterone. Consider the advantages and limitations of each and decide which method is most suitable to your research.
| Method | Advantages | Limitations |
|---|---|---|
| ELISA |
|
|
| RIA |
|
|
| GC-MS |
|
|
What methods do you recommend to manipulate sex hormones in male and female mice and rats?
Once a sex difference is known, there are several methods to determine if the effect is driven by sex hormones:
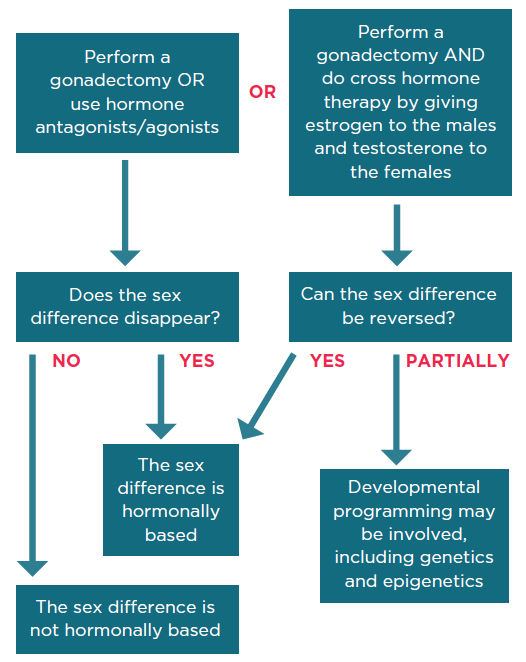
Long description
- Perform a gonadectomy OR use hormone antagonists/agonists
- Does the sex difference disappear?
- If yes, then the sex difference is hormonally based
- If no, then the sex difference is not hormonally based
- Does the sex difference disappear?
OR
- Perform a gonadectomy AND do cross hormone therapy by giving estrogen to the males and testosterone to the females
- Can the sex difference be reversed?
- If yes, then the sex difference is hormonally based
- If partially, then developmental programming may be involved, including genetics and epigenetics
- Can the sex difference be reversed?
What protocol do you recommend to recapitulate the estrous cycle in rats or mice following gonadectomy?
Wait one week after gonadectomy before administering exogenous sex hormones. Circulating hormones will be gone within 24 hours, but there should be enough time for hormones to be removed from the tissues. Don’t wait too long or the receptors will be disturbed! The specific protocol depends on whether you are using mice or rats, and which phase of the estrous cycle you are interested in replicating.
TIP: Antagonists to gonadotrophin releasing hormone (eg. Acyline) or estrogen (eg. Tamoxifen) are suitable alternatives to gonadectomy!
Rats
For rats, subcutaneous injections can be given over a couple of days to reasonably recapitulate the estrous cycle. To measure diestrus, inject 0.3 µg of estradiol benzoate a few hours before the experimental endpointFootnote 3. To measure proestrus, inject between 1-10 µg of estradiol benzoate a few hours before the experimental endpointFootnote 3Footnote 4. If you want to maintain a simulation of the estrus cycle for an extended period, administer 2 µg estradiol benzoate every four days. To measure sexual stimulation or behaviour, this requires 10 µg estradiol benzoate, two days in a row. Then skip a day and give one injection of 1 mg progesterone and test four hours later, making sure to test in the afternoon of the dark phase of the cycleFootnote 5.
Mice
With mice, it is not possible to use injections to mimic the estrous cycle. They must be implanted with a capsule subcutaneously that will release estradiol continuously for a week or two to get chronically high estradiol levels before they become sexually receptive. This would indicate if the effect is mediated by estrogen. If it’s not, then add a progesterone injection on top of the chronically high estrogen level to see if the effect is mediated by progesterone. The progesterone will have an effect within four to six hours.
What do you recommend as the best method of delivery for administering exogenous hormones to mice and rats?
There are several approaches that can be used. Consider the advantages and limitations of each and decide which method is most suitable to your research.
| Method | Advantages | Limitations |
|---|---|---|
| Injection |
|
|
| Pellet |
|
|
| Silastic Implant |
|
|
What other advice can you provide basic scientists?
Keep in mind that good old-fashioned bioassays can be used as surrogate markers of sex hormone levels.
| For males, seminal vesicle weight can be a good indicator of androgen levels. The higher the weight, the higher the androgen levels. | For females, uterine weight can be used as an indicator of estrogens in non-pregnant mice or rats. The higher the weight, the higher the estrogen levels. |
The views expressed in this document are those of Margaret McCarthy and do not necessarily reflect those of the CIHR Institute of Gender of Health or the Government of Canada.
- Date modified: