COVID-19 May 2020 Rapid Research Funding Opportunity Results
(2021-02-19): A collaboration with the COVID-19 Immunity Task Force (CITF) resulted in the additional funding of thirteen grants relevant to the CITF mandate in addition to funding top-ups provided to nine already funded applications. Additionally, Alberta Innovates also leveraged the May 2020 COVID-19 competition and separately funded seven project from Alberta-based research institutions (one of which was co-funded with CITF).
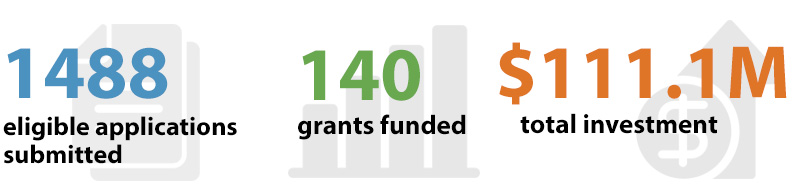
(2020-08-10): Following a recent application process review, an additional grant was funded, bringing the total number of funded grants to 140 and a total investment of $111.1 million.
Funding for the competition is provided through the Canadian Institutes of Health Research (CIHR), the International Development Research Centre (IDRC), the Michael Smith Foundation for Health Research (British Columbia), Alberta Innovates, Research Manitoba, Research Nova Scotia, the Saskatchewan Health Research Foundation, and the New Brunswick Health Research FoundationFootnote 1.
| Partner | Contribution |
|---|---|
| Canadian Institutes of Health Research* | $104,072,553* |
| International Development Research Centre | $6,554,078 |
| Michael Smith Foundation for Health Research | $150,000 |
| Alberta Innovates | $100,000 |
| Research Manitoba | $100,000 |
| Research Nova Scotia | $100,000 |
| Saskatchewan Health Research Foundation | $50,000 |
| * Includes funding from the CIHR Institute of Aging and contributions from the Government of Canada. | |
Peer review process
The peer review process relied on the time and dedication of experts across the health research spectrum. For this competition, a total of 926 individuals reviewed applications as part of the peer review process. Due to the urgent nature of this competition, an expedited review process was employed with one stage of virtual peer review. Peer reviewers declared their conflicts and ability to review and were assigned to ensure that there was an appropriate expertise on each application. Applications were assigned to four reviewers and the significant majority (99%) of reviewers had a high or medium declared expertise on their assigned applications. In the few cases where low expertise was assigned, it was a maximum of one reviewer per application.
Reviewers submitted an anonymized written review with strengths and weaknesses of the application in relation to the evaluation criteria as well as an overall rating. Due to COVID-19, there were no face to face meetings, however, score discrepancies where there could be a material impact on the application or competition outcome were reconciled. In order to be flagged for a discrepant review, an application must have been in the 25th percentile of applications, had a difference of 1.4 or greater between its highest and lowest score (the most extreme 25th percentile), and had at least 2 reviewers score the application above the projected research area funding cut off. These discrepancies were reconciled via videoconference where the assigned reviewers discussed the application and their individual ratings. During these calls, reviewers had the opportunity to amend their original scores based on the conversation. Once complete, final ratings were averaged to determine the final scores and rank ordered lists were generated for each research area.
Results: Across the Board Cut & Equalization
Due to the number of scientifically excellent projects as determined by peer review, CIHR made the decision to apply a 15.5% budget cut to all grants with an annual budget above $100,000, an approach commonly used in some other large programs (e.g., Project Grant Program). This budget cut allowed additional scientifically meritorious grants to be funded. This decision was also informed by the size of the grants awarded and their short duration.
Additionally, as outlined in the Tri-Agency Statement on Equity, Diversity and Inclusion, CIHR is committed to creating an equitable funding system by identifying and eliminating systematic biases towards any individual or group that would hinder access to CIHR funds. In this competition, CIHR has treated applications related to Indigenous Health Research (IHR) and applications submitted in French as separate cohorts. This approach has ensured that:
- IHR applications make up a minimum of 4.6% of the total competition budget. This is one of the commitments in CIHR’s Action Plan on Building a healthier future for First Nations, Inuit, and Métis Peoples.
- The proportion of applications submitted in French is approximately equal to the proportion of grants funded in support of the Official Languages Act.
All applications funded through the equalization were in the top 15% of the competition.
Results by research area
Applications were submitted to one of the following research areas: vaccines, diagnostics, therapeutics, clinical management and health system interventions, or social policy and public health response.
| Province | Number of applications submitted | Percent of total applications submitted | Number of applications funded | Percent of applications funded |
|---|---|---|---|---|
| Alberta | 192 | 12.9% | 21 | 15.0% |
| British Columbia | 146 | 9.8% | 20 | 14.3% |
| Manitoba | 48 | 3.2% | 6 | 4.3% |
| New Brunswick | 4 | 0.3% | 0 | 0% |
| Newfoundland and Labrador | 17 | 1.1% | 0 | 0% |
| Nova Scotia | 58 | 3.9% | 5 | 3.6% |
| Ontario | 673 | 45.2% | 62 | 44.3% |
| Prince Edward Island | 2 | 0.1% | 0 | 0% |
| Quebec | 312 | 21.0% | 21 | 15.0% |
| Saskatchewan | 36 | 2.4% | 5 | 3.6% |
| Total | 1488 | 100% | 140 | 100% |
- 8 of the 77 vaccine applications were funded with an average grant size of $1.47M.
- 16 of the 191 diagnostics applications were funded with an average grant size of $747K.
- 30 of the 339 therapeutics applications were funded with an average grant size of $1.17M.
- 37 of the 438 clinical management and health system interventions applications were funded with an average grant size of $747K.
- 49 of the 443 social policy and public health responses were funded with an average grant size of $503K.
Results were balanced across each of the research areas. There is not an equal number of applications funded within each research area because of the differing application pressure, however, the proportion of grants funded is approximately equal to the proportion of applications within all of the research areas.
| Research Area | Number of Applications Submitted | Proportion of Total Applications Submitted | Number of Funded Applications | Proportion of Total Applications Funded | Funding | Proportion of Total Funding |
|---|---|---|---|---|---|---|
| Vaccines | 77 | 5.2% | 8 | 5.7% | $11,788,552 | 10.6% |
| Diagnostics | 191 | 12.8% | 16 | 11.4% | $11,944,828 | 10.7% |
| Therapeutics | 339 | 22.8% | 30 | 21.4% | $35,134,942 | 31.6% |
| Clinical Management and Health System Interventions | 438 | 29.4% | 37 | 26.4% | $27,629,888 | 24.9% |
| Social Policy and Public Health Responses | 443 | 29.8% | 49 | 35.0% | $24,628,421 | 22.2% |
| Total | 1488 | 100% | 140 | 100% | $111,126,631 | 100% |
| Applicant self-identification dataFootnote 2 | ||||||
| NPIs who self-identified as woman | 579 | 38.9% | 62 | 44.3% | $39,519,410 | 35.6% |
| NPIs who self-identified as a member of a visible minority | 444 | 29.8% | 36 | 25.7% | $31,474,245 | 28.3% |
| Other Application Details | ||||||
| Clinical Trials | 214 | 14.4% | 19 | 13.6% | $36,180,809 | 32.6% |
| Indigenous Health Research (IHR) Applications | 33 | 2.2% | 9 | 6.4% | $5,267,212 | 4.7% |
| Applications submitted in French | 40 | 2.7% | 4 | 2.9% | $1,323,007 | 1.2% |
- Date modified: