What are clinical trials?
CIHR defines a clinical trial as a research study involving human participants that evaluates the safety and/or effects of one or more interventions on health outcomes.
Interventions include, but are not limited to, drugs, vaccines, radiopharmaceuticals, cells and other biological products, surgical procedures, radiologic procedures, devices, genetic therapies, natural health products, process-of-care changes, preventive care, manual therapies, and psychotherapies.
Data from clinical trials can be used to support the approval of drugs for Canadians or to compare different medicines or treatments. The data can also help us determine which treatments are best for specific populations.
Clinical Trials at CIHR
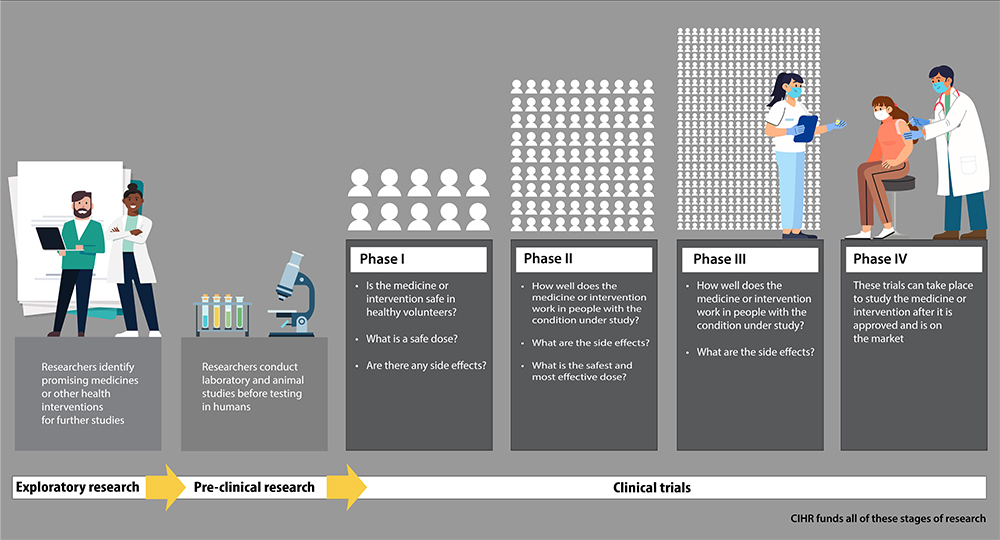
Infographic description
Exploratory research
Researchers identify promising medicines or other health interventions for future studies.
Pre-clinical research
Researchers conduct laboratory and animal studies before testing in humans.
Clinical trials: Phase 1
Is the medicine or intervention safe in healthy volunteers?
What is a safe dose?
Are there any side effects?
Clinical trials: Phase 2
How well does the medicine or intervention work in people with the condition under study?
What are the side effects?
What is the safest and most effective dose?
Clinical trials: Phase 3
How well does the medicine or intervention work in people with the condition under study?
What are the side effects?
Clinical trials: Phase 4
These trials can take place to study the medicine or intervention after it is approved and is on the market.
CIHR funds all these stages of research.
- Date modified: