CIHR-CEPI Leadership Award for Excellence in Vaccine Research for Infectious Disease of Epidemic Potential
Figure 1. CEPI Vaccine Research and Development Activities
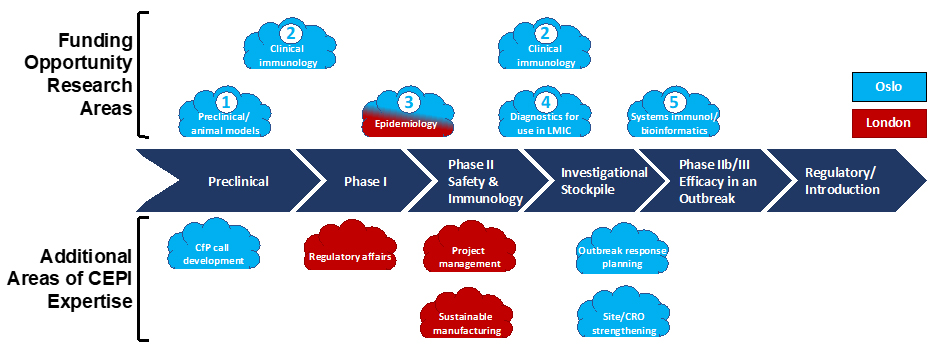
CfP: call for proposals; CRO: clinical research organization; LMIC: low- and middle-income countries.
Figure 1 – Long description
CEPI research and development (R&D) activities in Oslo, Norway are shown in light blue, and activities in London, UK are shown in red, although some research activities may occur at either location. CIHR and CEPI are looking to support talented Canadian mid-career researchers to work with the R&D group based in either location. Research areas for this funding opportunity are shown above the vaccine development timeline (top portion of the figure) and include: (1) Preclinical development and animal models, (2) Clinical immunology, (3) Epidemiology, (4) Diagnostics for use in LMIC, and (5) Systems immunology and bioinformatics. Additional areas of CEPI expertise are shown below the timeline (bottom portion of the figure). While these are not part of the funding opportunity, they are areas you may be exposed to during your co-developed project with CEPI.
Figure 2. Application Process, Project Co-Development and Reporting Overview*
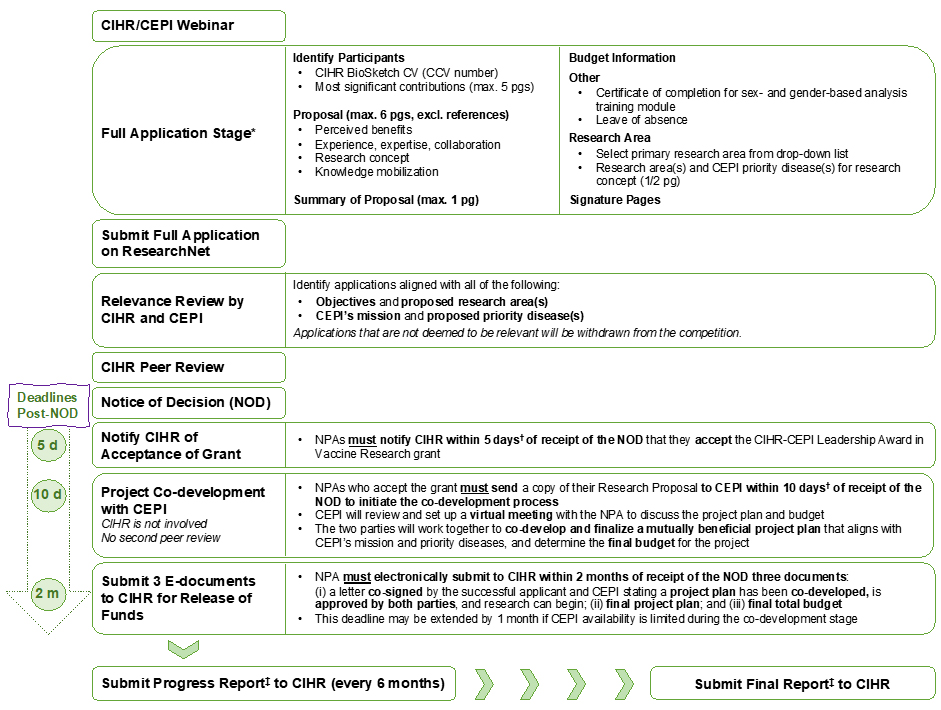
* Refer to the funding opportunity text for additional details on the application stage, co-development process, reporting requirements, and conditions of funding;
┼ Business days;
‡ Details of progress and final reports will be provided by CEPI and CIHR. An electronic Final Report will be made available to the NPA on ResearchNet at the beginning of the grant funding period and can be filled in as the research progresses.
CCV: Canadian Common CV; d: days; excl.: excluding; m: months; Max: maximum; NOD: Notice of Decision; NPA: nominated principal applicant; pg: page; pgs: pages.
Figure 2 – Long description
This figure provides an overview of the application, project co-development and reporting stages for this funding opportunity. CIHR will be hosting a webinar with the Coalition for Epidemic Preparedness Innovations (CEPI) to support participants with the requirements of this funding opportunity and to answer questions. The application process for this funding opportunity is comprised of one step: Full Application.
Specific instructions to submit an application:
- Identify Participants: the NPA is required to (i) submit a CIHR Biosketch CV. All CVs must be entered using the Canadian Common CV confirmation number; (ii) describe your most significant contributions as they relate to the application (maximum of 5 pages).
- Proposal Information section: The proposal is limited to six (6) pages, not including references. The use of proposal headers to facilitate peer review is required: (i) Perceived Benefits for the Applicant’s Career Development and for CEPI; (ii) Experience, Expertise and Collaboration; (iii) Research Concept; (iv) Knowledge Mobilization.
- Summary of the Proposal (maximum 1 page)
- Enter Budget information
- Other: attach (i) Certificate of Completion for the sex- and gender-based analysis training module (mandatory); (ii) Leave of Absence (mandatory if applicable).
- Select Research areas: (i) select your primary research area from the drop-down list; (ii) clearly identify and describe (in one half-page) how the research proposed will address the relevant research area and clearly identify the CEPI priority disease(s) for your proposed research concept.
- Submit application on ResearchNet.
- Relevance review for CIHR and CEPI: Identify application aligned with: (i) objectives and proposed research area(s); and (ii) CEPI’s mission and priority disease(s). Applications that are not deemed to be relevant will be withdrawn from the competition.
- CIHR peer review process: Peer review will be conducted in accordance with the Review Guidelines – Priority-driven Initiatives
Notice of Decision (NOD) and important deadlines
- Notify CIHR of Acceptance of Grant. The NPA must notify CIHR they have accepted the CIHR-CEPI Leadership Award in Vaccine Research grant within five (5) business days┼ of the receipt of the Notice of Decision
- Project co-development with CEPI (CIHR is not involved; no second peer review). Successful applicants who accept the grant are required to send a copy of their Research Proposal to CEPI within ten (10) business days┼ of the receipt of the Notice of Decision to initiate the co-development process for the research project. CEPI will review and set up a virtual meeting with the NPA to discuss the project plan and budget. The two parties will work together to co-develop and finalize a mutually beneficial project plan that aligns with CEPI mission and priority disease and determine the final budget for the project.
- Submit 3 E-documents to CIHR for release of funds. The NPA must electronically submit to CIHR three (3) documents: (i) a letter co-signed by the NPA and CEPI that confirms that a detailed project plan has been co-developed and is approved by both parties, and research can begin; (ii) a copy of the final project plan; and (iii) a copy of the final total budget. This deadline will be extended by one month if CEPI availability is limited during the co-development stage.
- Submit Progress report** to CIHR
- Submit Final report** to CIHR
- Date modified: