
What We Heard Report: Future of Clinical Trials
What We Heard Report: Future of Clinical Trials
Related
Introduction
The Canadian Institutes of Health Research (CIHR) is committed to strengthening the clinical trials ecosystem to improve health care and health outcomes for all Canadians. CIHR is now looking to build a long-term strategy to support Canada’s clinical trials ecosystem. In the fall of 2022, CIHR launched Building the future of clinical trials, an online consultation with the research community, partners, and stakeholders involved in clinical trials. Participants were asked questions about CIHR’s role in funding clinical trials and in developing related policies and programs. Their discussions and preliminary recommendations will inform our ongoing conversations in order to shape the development of a long-term clinical trials strategy.
CIHR received a total of 44 responses from researchers, patient and citizen groups, research and health networks, government departments, and several organizations between September 28, 2022, and December 1, 2022. These 44 responses represent the collated comments from over 140 individuals, representing over 100 organizations or groups of stakeholders.
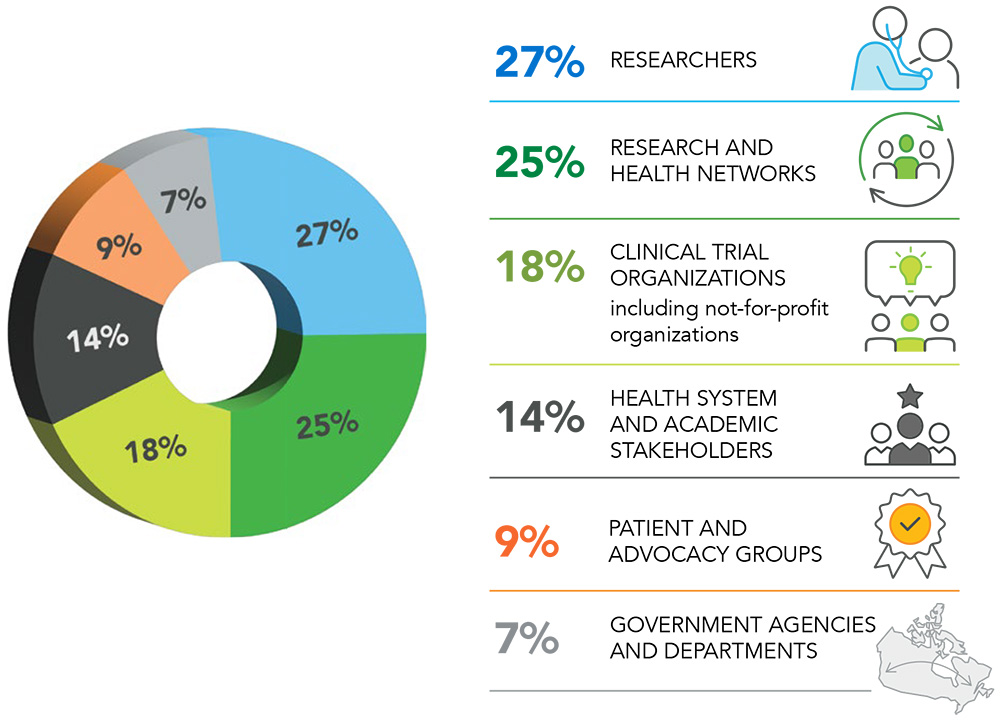
Figure 1. Stakeholders who provided feedback
Long Description
These 44 responses represent the collated comments from over 140 individuals, representing over 100 organizations or groups of stakeholders. Of these 44 responses: 27% came from researchers, 25% came from research and health networks, 18% came from clinical trial organizations including enabling and not-for-profit organizations, 14% came from health system and academic stakeholders, 9% came from patient and advocacy groups and 7% came from the government agencies and departments.
Highlights
Participants identified the key challenges in conducting clinical trials in Canada. They also provided innovative and sustainable solutions and shared lessons learned from international clinical trial models:
-
Identified barriers to advancing clinical trials in Canada
- Duplication of processes and considerable time and resources needed for start-up, such as inefficiencies in ethics reviews, institutional approvals and regulatory review for multisite trials, and complicated contract requirements.
- Complexities in CIHR funding application processes.
- Lack of infrastructure support for clinical trial programs, which adds administrative and operational burden to health organizations.
-
Recommendations about improving CIHR funding for clinical trials
- Create a pan-Canadian structure that coordinates efforts, reduces duplication and takes into account existing infrastructure (clinical trial networks, SPOR SUPPORT Units).
- Expand clinical trial funding to investigators and health care units outside of academic institutions and provide sustainable funding and training infrastructure for clinical trial programs to enable retention of highly qualified personnel.
- Dedicate funding for the full drug development pipeline from early discovery research to post-clinical trial processes.
- Allow sufficient time and streamlined administrative requirements to form patient partnerships, and provide guidance and policies around academic-industry partnerships to advance made-in-Canada health innovations.
-
Suggestions for future direction of funding for innovative clinical trials
- Include adaptive platform trials and master protocols (flexible designs allowing rapid adaptation to new knowledge and the availability of potential treatments), cluster randomized trials and cohort studies that are collecting real-world evidence.
- Work towards a health data system that maintains privacy while allowing research access and data sharing.
- Support decentralized clinical trials that use technological innovations that enable remote study visits to increase access to clinical trials for all Canadians.
-
What we heard about clinical trial priorities within Canada’s Biomanufacturing and Life Sciences Strategy
- Expand the breadth of support to include pre-clinical and translational research, and prioritize funding of early (pilot, phase I, phase II) trials to grow Canada’s drug development and biomanufacturing sector.
- Ensure sustainable, ongoing funding for core clinical research staff and support for specialized infrastructure such as phase I clinical trial facilities in proximity to health care settings and domestic Good Manufacturing Practice facilities.
- Support the capability to manufacture investigational products and medical devices within Canada via academic-private partnerships.
- Provide training specific to clinical trials for investigators in the areas of study design and biostatistics, ethics, data privacy, regulatory processes, cultural safety, translational research methods and meaningful patient and stakeholder engagement.
-
Advice on CIHR policies to support equity, diversity and inclusion, transparency, and research excellence
- Increase the diversity of participants in clinical trials, such as inclusion of children, people with disabilities and social vulnerabilities, equity-seeking groups, and groups that are underrepresented in science.
- Collect demographic information of clinical trial participants and require transparency of disaggregated data.
- Mandate patient partnerships and facilitate the connection of clinical trial teams with organizations that have expertise in patient engagement (for example, SPOR).
- Support innovations such as decentralized trials for open and equitable access for diverse populations.
- Create equity, diversity and inclusion evaluation metrics and standards and require meaningful patient-reported outcomes in clinical trial applications.
- Ensure an Indigenous engagement strategy.
-
Key elements to consider including in a long-term clinical trials strategy
- Integrate clinical trials within the health care system with infrastructure support for clinical trial programs and highly qualified personnel.
- Coordinate clinical trial start-up activities (ethics, contracts), data sharing mechanisms, and research transparency (standardized application templates, minimal-consent guidelines, agreement templates, etc.) at the national level.
- Enable the inclusion of all Canadians in clinical research.
- Develop meaningful patient engagement when establishing research priorities and developing clinical trial protocols so that the outcome of the research meets the needs of patients.
- Foster CIHR leadership in clinical trials at a national and international level.
- Build long-term funding streams with predictable cycles of grant calls and sufficient time to develop strong applications.
- Increase the capacity of highly qualified personnel to perform clinical trials by providing training programs in trial design and biostatistics, regulatory compliance, quality assurance and clinical research administration.
-
Recommendations for learning from best practices in other countries
- The United Kingdom’s National Institute for Health and Care Research (NIHR) is a good model for infrastructure funding and knowledge translation.
- The US National Institutes of Health (NIH) offers many programs that can be modelled, such as the NIH Planning Grant Program, National Center for Advancing Translational Sciences, Accelerating Medicines Partnerships, Patient-Centered Outcomes Research Institute, etc.
- Research priority-setting should be an ongoing process that takes into consideration multiple and diverse perspectives, including the health priorities of Canadians, the impact of the research on the health system, and the coordination of research efforts. The NIHR’s James Lind Alliance was frequently cited as a high-quality model.
Where to go next
Clinical trials are an essential research tool for optimizing patient care that requires the coordination of scientific, clinical, and administrative processes and input from multiple and diverse perspectives. This is why these insights matter, and we would like to thank all participants for their responses. Their input will guide future conversations about identified gaps and inform a long-term clinical trials strategy to support clinical trials research, training, and infrastructure in Canada.
For additional information, please contact clinicaltrials-essaiscliniques@cihr-irsc.gc.ca.
- Date modified: