Internal Assessment for 2011 International Review - CIHR Institute of Infection and Immunity
Table of Contents
- Mandate and Context
- Institute Priorities
- Key Initiatives
- Outputs and Outcomes
- Going Forward
- List of Acronyms and Abbreviations
- References
List of Figures and Tables
- Figure 1: III mandate expenditures and number of grants by fiscal year
- Figure 2: Percentage of total CIHR expenditures related to III mandate areas over time
- Figure 3: Specialization index and average of relative citations for top 10 countries publishing in antibiotic resistance and infection control, 2000-2008
- Figure 4: Co-publications among members of funded teams in the Safe Food and Water Initiative
- Table 1: III research priorities of the 2002-2007 strategic plan
Mandate and Context
Mandate and mission
Created in 2000 as one of the 13 institutes of the Canadian Institutes of Health Research (CIHR), the Institute of Infection and Immunity (III) has a mandate to support research to enhance immune-mediated health and to reduce the burden of infectious disease, immune-mediated disease and allergy through prevention strategies, screening, diagnosis, treatment, support systems and palliation. The III mandate transcends disciplines and encompasses all four health research themes: biomedical; clinical; health systems and services; and social, cultural and environmental factors that affect the health of populations. III's mission is to establish national leadership, priorities and programs to reduce the global burden of infection and immune-based diseases.
Structure and operations
The Institute has a larger Ottawa-based staff than other CIHR institutes to manage the additional responsibilities of the HIV/AIDS, hepatitis C and pandemic preparedness initiatives. In December 2009, inaugural Scientific Director Dr. Bhagi Singh was succeeded by Dr. Marc Ouellette and the Institute relocated to Université Laval in Québec City. The Institute is guided in its activities by an Institute Advisory Board (IAB), which meets three times a year and is comprised of leading national and international III researchers, representatives of partner organizations, industry and an ethics designate.
Additional funding for III
Hepatitis C
In addition to the normal Institute strategic initiative budget of $8.5 million per year, III manages close to $1 million per year to support hepatitis C research. The Hepatitis C Research Initiative began in 1999 as a joint five-year initiative between the Medical Research Council and Health Canada, with additional funds provided to CIHR in 2004 and 2006. In 2008, the federal government renewed the program with an ongoing commitment of $900,000 per annum to support biomedical, clinical and psychosocial/ behavioural research and training.
Pandemic preparedness
In 2006, III assumed the management of the $21.5 million over five years awarded to CIHR by the Government of Canada to support pandemic influenza research. III has now increased this funding envelope to $45.7 million, through partnerships with the Public Health Agency of Canada (PHAC), the Canadian Food Inspection Agency, Alberta Innovates - Health Solutions and Canada's Research-based Pharmaceutical Companies.
The CIHR HIV/AIDS Research Initiative
III also manages $22.5 million per year in ongoing funding to support the CIHR HIV/AIDS Research Initiative. The Government of Canada has a 20-year history of supporting national HIV/AIDS strategies such as the Federal Initiative to Address HIV/AIDS in Canada and, more recently, the Canadian HIV Vaccine Initiative. The Canadian Medical Research Council was a partner in these strategies from the beginning, managing the funding for biomedical and clinical research. Since the transition to CIHR in 2000, additional funding streams have been transferred to CIHR including the Canadian HIV Trials Network (CHTN), health services/population health funding, and the HIV/AIDS Community-based Research Program. The CIHR HIV/AIDS Research Initiative is managed by a small Ottawa-based team guided by the III scientific director and the CIHR HIV/AIDS Research Advisory Committee (CHARAC), which is chaired by a member of the Institute's IAB.
The Institute has additional responsibilities through the management of the HIV/AIDS, hepatitis C and pandemic preparedness initiatives.
The Canadian context - research funding in infection and immunity
The Institute was created within a strong, well-established research community in both immunology and infectious diseases that was particularly active in biomedical research but less so in the domains of clinical, health services and population health research. Figure 1 shows a steady increase in both the number of grants and the total expenditures for open and strategic funding streams in the infection and immunity domain from 2000-2001 to 2007-2008, followed by a plateau that reflects a period of reduced growth in the overall CIHR budget and individual institute strategic research budgets.
Figure 1: III mandate expenditures and number of grants by fiscal year
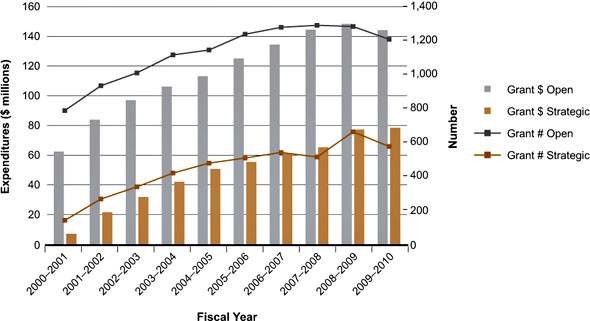
Data in Figures 1 and 2 are based on a keyword search of the CIHR funding database and validated through a subjective process. Projects may have multiple institute affiliations.
Represented as a proportion of CIHR funds, Figure 2 indicates a thriving research community that accounts for roughly 30% ($250 million) of the current CIHR grants and awards budget.
Figure 2: Percentage of total CIHR expenditures related to III mandate areas over time
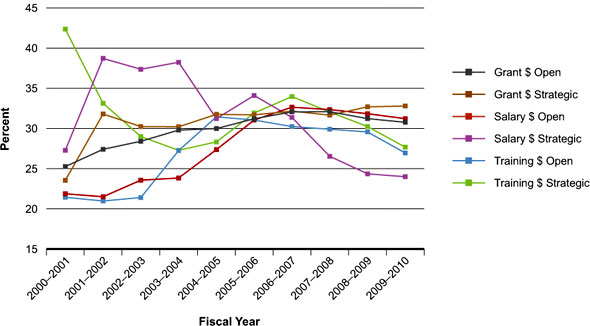
The fluctuations in percentages since 2000-2001 (Figure 2) reflect changing CIHR strategies for salary and training awards over time and the modest CIHR investment in overall strategic funding prior to the creation of the institutes. However, in 2000-2002, preceding the creation of the institutes, there was little strategic CIHR investment other than in the HIV/AIDS and hepatitis C initiatives. This is reflected in the high percentages of III-related funding for strategic salary and training awards during this period.
Recommendations from the 2006 CIHR International Review
In the 2006 CIHR International Review, III was commended for promoting research excellence and building capacity, and for its partnerships with federal and provincial governments, research organizations, non-governmental and international organizations. The Institute was praised for its efforts in addressing knowledge translation (KT), although the review panel noted that further work was required in this area. The Institute was also encouraged to strengthen communications with the research community and the general public. The panel recommended that effective performance targets be identified but stated that, overall, III appeared to be on the right track and moving towards achieving its mandate and objectives. In fact, a 2005 EKOS survey of a sample of health researchers found that 91% of researchers felt III had been "very" or "to some extent" successful in influencing the research agenda within its mandate.
Institute response to the 2006 International Review
In response to the 2006 review, III now produces and distributes regular newsletters and communiqués describing the Institute's activities and funding opportunities. Due to the impact of issues such as HIV/AIDS, severe acute respiratory syndrome (SARS) and porcine influenza (H1N1) in the last five years, media coverage has been prominent and III is often cited as one of the top three institutes for media mentions in each quarter. Institute staff attends and presents at national and international meetings related to the III mandate, often with the CIHR booth. This provides a venue for distributing Institute publications and for interacting with the research community.
Many of III's initiatives have a strong KT component. For example, CIHR funding for targeted KT activities related to HIV/AIDS research has ramped up steeply, from seven grants ($185,000) in 2005-2006 to 29 grants ($780,000) in 2009-2010. In 2009, III organized a workshop to determine the potential scope and mechanisms for implementing a KT strategy for the Institute. The workshop reportFootnote 1 charts a path for improving efforts in this important area.
Institute Priorities
The Institute's strategic plans
Given the existing well-established and well-funded infection and immunity community, an initial challenge for III was to identify gaps and opportunities where strategic initiatives would likely have a significant impact. The Institute's first strategic plan, 2002-2007, was developed in collaboration with the research community, professional societies, non-profit agencies, patient groups and the Institute Advisory Board (IAB). Consultations took place through numerous town hall meetings at Canadian academic centres, small working group sessions with key research leaders, thematic workshops and an e-mail survey of the III community at large. In consultation with the IAB, III identified two broad strategic research themes with several related priorities grouped under each (Table 1). A key overarching priority for III has always been training and capacity building. The first slate of priorities shows a good balance between the two components of the III mandate: infection and immunity. The Institute has developed strategic initiatives to address all of these priority areas.
Table 1: III research priorities of the 2002-2007 strategic plan
| Infectious Diseases | Host Response |
|---|---|
| Antimicrobial resistance | Asthma and allergy |
| Emerging infectious diseases | Autoimmune diseases |
| HIV/AIDS and hepatitis C | Innate immunity |
| Safe food and water | Organ transplantation and tissue regeneration |
| Novel vaccine development |
Following a second round of consultations, the Institute's second strategic plan (2007-2012) regrouped the original priorities to achieve greater focus:
- Emerging infections and microbial resistance - solutions from innovations in tools and technologies
- HIV/AIDS - from prevention and therapy to addressing global health challenges
- Immunotherapy - new approaches through systems biology
- Pandemic influenza preparedness - prevention, therapy and public health challenges
- Vaccines of the 21st century - integrating innate and adaptive immunity and novel vaccine technologies
The III challenge - Responding to emerging threats
From the outset, III was challenged with a series of emerging health threats including: the E. coli outbreak in Walkerton, Ontario; bioterrorism threats following the attacks of 9/11; severe acute respiratory syndrome (SARS); bovine spongiform encephalopathy (BSE); West Nile virus; Clostridium difficile outbreaks; avian influenza (H5N1); porcine influenza (H1N1); and an alarming increase in antibiotic resistance. The Institute has been proactive in responding rapidly to these threats through a variety of strategic initiatives. Pandemic influenza preparedness became an Institute priority following the SARS outbreak in 2003 and the Influenza Research Priorities Task Group was assembled to identify strategic priorities. The priorities were: vaccines and immunization programs, the virus, biology and rapid diagnostics, prevention and treatment, ethics, legal and social research and capacity building.Footnote 2
The Institute has identified strategic research priorities in both immunology and infectious diseases while retaining the flexibility to address emerging priorities precipitated by sudden health crises.
Setting priorities for HIV/AIDS
For the CIHR HIV/AIDS Research Initiative, III has transformed a collection of disjointed programs into a comprehensive strategic approach to HIV/AIDS research in Canada. In 2003, III created the CIHR HIV/AIDS Research Advisory Committee (CHARAC), to provide strategic directions. CHARAC members represent multiple CIHR institutes, various HIV/AIDS research themes, government and HIV/AIDS community organizations. In 2007-2008, CHARAC led a consultation process to develop the first strategic plan for the Initiative.Footnote 3 This plan sets out six priority research themes: health systems, services and policy; resilience, vulnerability and determinants of health; prevention technologies and interventions; drug development, toxicities and resistance; pathogenesis; and issues of co-infection and co-morbidity. The HIV/AIDS Initiative and its strategic plan support Canada's longstanding strength and expertise in biomedical HIV research and foster the development of further expertise and research capacity across other research domains.
Key Initiatives
As the Institute's core strategic budget of $8.5 million per year represents only 3.4% of the 2009-2010 CIHR investment in infection and immunity research, III realized from the outset the importance of strategic planning if it was to have any impact. As the vast majority (approximately 70%) of CIHR funding in the III domain supports biomedical research, III has focused on building on the strength of this community and directing their efforts towards areas of identified need. The Institute's strategic initiatives have also built capacity in the other three research themes: clinical, health services and population health. Since 2000, III has developed strategic initiatives focused on infectious diseases and immunology. In many cases III has bridged the gap between these communities by actively promoting collaborations between immunologists and microbiologists in such areas as vaccines, microbial resistance and innate immunity. For example, III launched an initiative targeting mucosal immunity following the SARS outbreak ($4.5 million) and recently made a major investment ($10 million) in clinical autoimmunity. The key initiatives described below were selected based on the availability of measurable outcomes and because they highlight III's success in recognizing and responding to emerging health threats, managing and coordinating large, politically charged, federal research agendas, and building capacity in important but underserved research areas.
Initiative 1: Emerging threats
Safe food and water
When E. coli-contaminated water killed nine people and sickened hundreds in Walkerton, Ontario in 2000, food and water contamination became a public and government priority in Canada. The formation, by III, of the 17-member Canadian Research Coalition for Safe Food and Water in 2002, heralded a new era of cooperation between federal departments, funding agencies and industry associations - something that would have been difficult to achieve prior to the creation of III. The coalition identified national research priorities and developed a process for combining funds and resources to jointly address a shared health concern that crossed the mandate of many organizations. The group went on to support an environmental scan, seven multidisciplinary, multi-sector teams for an investment of more than $5 million over three years,Footnote 4 and an innovative knowledge translation program in the form of a traveling museum exhibit, "Food for Health", which explores food safety and the links between diet and health.Footnote 5 The emergence of food safety issues such as bovine spongiform encephalopathy (BSE) and listeriosis-contaminated meat emphasizes the importance of a continued focus on this topic.
III's leadership in the Safe Food and Water Initiative established a new era of collaboration between government departments and academia.
SARS
SARS was a new respiratory disease that emerged in China and spread rapidly around the world in 2003. By the end of the epidemic, 774 people had died, including 43 in Canada. In response, III formed the Canadian SARS Research Consortium - a group of representatives from national and provincial governments, the private sector and health associations -to coordinate and support research on diagnostics, vaccine development, therapeutics, epidemiology, databases, public health and community impact. III also established the Canadian Rapid Research Response Team, a virtual network of Canadian and international partners to plan for future outbreaks. This innovative, agenda-setting approach would have been nearly impossible before the creation of CIHR and was largely achieved by III's highly committed scientific director and dedicated staff.
The Institute set an all-time record at CIHR by launching and reviewing a funding opportunity in 19 days without compromising peer review. This rapid response received national and international attention and was cited by the federal government as an example of excellence in Canadian innovation.Footnote 6 Between April 2003 and April 2005, III and partners launched four strategic initiatives to support research on the biology and epidemiology of SARS, the impacts of the outbreak on public health and health systems, and to develop a SARS sample bank and registry to support future research. The Institute contributed $250,000 of its own development grant to support these initiatives, which grew to $1.7 million through partnerships. Independent evaluations have since been done on the SARS Research ConsortiumFootnote 6 and CIHR's response to SARS.Footnote 7 In the words of one of the partners:
"CIHR-III showed great leadership and a sense of urgency in bringing together the scientific community in Canada from academia, public health and industry to respond to the SARS crisis."
Pandemic influenza
In 2006, prompted by the outbreaks of SARS and H5N1 influenza, and the widespread belief among health authorities that another influenza pandemic was inevitable, the Government of Canada allocated funds to create the Pandemic Preparedness Strategic Research Initiative (PPSRI). A task group was established which, in consultation with stakeholders, developed a new set of outbreak-specific research priorities: epidemiology and natural history, biology of the virus and anti-virals, viral immune response and contributing co-factors, ethical issues, and vaccine development and evaluation. The research component of PPSRI, managed by III, has supported a broad range of funding opportunities to address these research priorities through operating grants, catalyst grants, team grants, applied public health chairs and meeting grants. The PPSRI also supported the formation of the PHAC-CIHR Influenza Research Network (PCIRN) to evaluate the pandemic vaccine in conjunction with provincial and municipal public health authorities and inform Canadian immunization procedures and policies during the H1N1 pandemic.
When the H1N1 outbreak occurred in the spring of 2009, this existing infrastructure and funding framework facilitated the rapid funding of catalyst grants targeting pandemic-specific topics. As part of this Initiative, III worked with the CIHR Ethics Office to develop an ethics process that could be employed in future public health emergencies. This led to the development of the Canadian Program of Research on Ethics in a Pandemic (CanPREP). The mid-term evaluation of PPSRI can be found on the Institute website.Footnote 8
Initiative 2: The CIHR HIV/AIDS Research Initiative
Almost 25 years after the discovery of HIV, millions of people around the world, including thousands of Canadians, are newly infected with HIV each year. Without a cure, millions continue to suffer and die from AIDS. The CIHR HIV/AIDS Research Initiative aims to reduce the burden of HIV/AIDS through the implementation of its strategic planFootnote 3 and a combination of targeted funding opportunities and priority announcements. Priority announcements support additional high-quality HIV/AIDS grants and awards submitted to open competitions and over the years have helped sustain a high level of research activity and foster capacity building on an ongoing basis across all identified priority areas. Targeted funding opportunities have been developed in the strategic areas of prevention, health systems and services, and resilience, vulnerability and determinants of health. The initiative further supports HIV/AIDS priorities through partnerships on a number of relevant funding opportunities led by other CIHR institutes.
The targeted funding for HIV/AIDS will increase further in the coming years due to the Institute's role in the Canadian HIV Vaccine Initiative (CHVI). The CHVI, a collaboration between the Government of Canada and the Bill and Melinda Gates Foundation, represents an enhanced Canadian contribution to global efforts to develop an HIV vaccine.Footnote 9 Combined CIHR and Canadian International Development Agency funding of $22 million will support the discovery of HIV vaccines and the related social and behavioural issues.Footnote 10
A unique perspective - Community-based research
The III-led CIHR HIV/AIDS Community-Based Research (CBR) Program engages communities in all stages of research, including at the definition of the research question, during the research process and at the dissemination of research results. With a budget of approximately $2.7 million per year, the program supports general and Aboriginal programs. Since the transfer of this program to CIHR in 2004, III has worked extensively to adapt CIHR systems to accommodate leading roles for non-academics. Through these efforts, knowledge users now have defined roles in the CIHR system and can hold CIHR funds for specific programs. As well, a merit review system has been created that rates proposals on their potential impact as well as scientific merit and engages knowledge users on the review panel. These changes ensure that knowledge transfer and research uptake are central to the research process. An evaluation of the HIV/AIDS CBR program in 2008-2009Footnote 11 concluded that the program is helping communities and academia respond to the HIV/AIDS epidemic, and is building research capacity at the community level and in academic circles. In the words of one researcher:
"The CIHR HIV/AIDS Community-Based Research Program has become a model for enabling communities to play a central role in HIV/AIDS-related health research that produces action-oriented outcomes. Whether it is people living with HIV/AIDS, Aboriginal groups, newcomers to Canada, or members of other at-risk communities, this innovative program supports research that provides solutions to knowledge users impacted by this epidemic, including people living with HIV/AIDS, policy makers and service providers. As such, it is a model of research that ensures 'knowledge to action' strategies for change."
Initiative 3: Antibiotic resistance
Antibiotic resistance has been an Institute priority from the outset and III has launched several strategic initiatives since 2001. One focused on vulnerable populations such as the elderly and people living in northern Canadian communities. Another examined links between antibiotic use in agriculture and antibiotic resistance in humans. A third centred on infection control practices. In 2006, III launched its largest initiative in antibiotic resistance: the Novel Alternatives to Antibiotics Initiative. The initiative focused on novel approaches such as immune system function and the potential to modulate antibacterial immune responses, the design of physical systems and biomaterials that can resist bacterial and biofilm growth, and innovative alternatives to antibiotics, such as phage and probiotics, that were not being funded in the CIHR open competition.Footnote 12 III launched the initiative with 26 partners, including three CIHR institutes, the CIHR Innovation and Industry branch, three agriculture associations, eight national and international companies, five charitable organizations, five government departments and one Network of Centres of Excellence (NCE). The initiative funded research fellowships, high-risk projects and multidisciplinary teams for a total financial commitment of close to $13 million.
The Institute's novel approach to antibiotic resistance attracted national and international partners, many of which were new to CIHR.
In addition, III arranged for Canadian researchers to attend a workshop in Moscow organized by the Department of Foreign Affairs and International Trade to explore partnership opportunities through the International Science and Technology Centre (ISTC) in Moscow. As a result, Dr. Sylvain Moineau is now a collaborator with four Russian/Georgian colleagues on phage therapy projects (one funded by ISTC), which gives him access to unique phage libraries.
Outputs and Outcomes
Advancing knowledge
Many advances in knowledge have taken place in the infection and immunity field, especially from the strong community of researchers funded through the CIHR open competitions. A few key examples include:
- the discovery by Dr. Andrew Macpherson of the mechanisms by which microbiota gravitate to a specific host organFootnote 13
- the role of microbiota in inflammatory bowel disease by Dr. Brett FinlayFootnote 14
- the role of specific cellular receptors in autophagy, a crucial defense mechanism against bacteria by Dr. Dana PhilpottFootnote 15
- the demonstration by Dr. Julio Montaner that contemporary antiretroviral therapeutic guidelines significantly reduce HIV transmissionFootnote 16
- the concept of resistome defining the collection of all antibiotic resistance genes in an ecosystem by Dr. Gerald WrightFootnote 17
- the identification by Dr. Rafick-Pierre Sekaly of a subset of CD4(+) T-cells that serve as a reservoir for HIV replication and persistence and its potential role as a target for viral eradicationFootnote 18
- the discovery by Dr. Tak Mak of the crucial role of the Fas receptor in the normal development of B- and T-cellsFootnote 19
Institute strategic initiatives are now also beginning to contribute new knowledge in the world literature, as described below.
Emerging infections
Data compiled for the Impacts of the CIHR Institute of Infection and Immunity, 2000-2008 document,Footnote 20 show a four-fold increase in the number of Canadian publications related to food and water safety between 2002 and 2007 following funding of the III Safe Food and Water Initiative. This same document describes a 30-fold increase in Canadian publications related to pandemic influenza between 2003 and 2007, which occurred largely as a result of publications on the SARS outbreak and the lessons learned. In fact, the SARS Impact Assessment document reports the creation of new knowledge in outbreak control, treatment, health policy and antivirals resulting in more than 100 publications and more than 300 conference presentations, including a Paper of the Year award in 2006 for the description of a novel antiviral for SARS.Footnote 6 As of May 2010, 84 articles have been published as a result of funding from the pandemic preparedness initiative. It is expected that this number will grow over time. Specific examples of knowledge creation include:
Preventing food- and waterborne-illness
Dr. Subash Sad and his team have shown that pathogens such as Salmonella typhimurium that proliferate rapidly and chronically in vivo evoke functionally inferior memory CD8+ T cells, which may promote the survival of the pathogen, opening the door for immune modulation.Footnote 21 Another study, by Dr. Mohamed Karmali's team on verotoxin-producing
E. coli (VTEC O157) bacteria serogroups associated with human illness, has identified key genomic islands associated with VTEC of high risk to human health.Footnote 22 This finding will lead to improved diagnosis and management of this highly dangerous food- and waterborne pathogen.
Preparing for the next pandemic
Outcomes from the pandemic preparedness initiative such as development of a recombinant live attenuated swine H1N1 influenza vaccine,Footnote 23 the identification of a compound that reduces viral production and protects cells from death due to Tamiflu-resistant influenza virus,Footnote 24 and the characterization of the transmission dynamics of the H1N1 virusFootnote 25 will all be important in the control of future influenza outbreaks.
The CIHR HIV/AIDS Research Initiative
Data compiled for the 2009 Impact Assessment of the HIV/AIDS Initiative shows that the number and quality of Canadian publications in HIV/AIDS has increased and the type of publication generated in Canada has evolved. Between 1996-1998 and 2006-2008, the number of such publications per year doubled, accompanied by a steep increase in the Canadian share of world publications. In 1996-1998 and 2006-2008 Canadian publications were the second-most cited in the world, suggesting our influence on HIV/AIDS research outweighs our relatively small 5% publication contribution. Canada has a strong history of biomedical HIV research and this field accounts for 60% of the country's HIV/AIDS publications. This is less than the 69% in the same field for 1996-1998, but the lower percentage is a consequence of increased productivity in other areas of HIV research. For example, between 1996-1998 and 2006-2008, social sciences publications increased from 8% to 13% of the total. This accompanied a dramatic increase in Canadian HIV/AIDS publications for community-based research (CBR), which has a world share almost twice as large as Canada's share of HIV/AIDS research overall. Even more striking is the increase in productivity of Aboriginal CBR for which the Canadian world share rose to 28% for 2005-2008 from 13% in 1996-2001.Footnote 11 These publication data exemplify how the face of HIV/AIDS research in Canada has changed over a decade through sustained and targeted investments across a wide variety of research disciplines.
Important discoveries in HIV/AIDS
Canada's contribution to international knowledge on HIV/AIDS has had a tremendous impact on the life expectancy of people living with the disease and is helping to slow the transmission of new infections. For example, Dr. Mark Wainberg demonstrated the anti-HIV properties of 3TC, a compound still in use today for the treatment of HIV.Footnote 26 Dr. Patricia Spittal and her team on the Cedar Project Partnership are uncovering the complex relationships between gender, sexual abuse, drug use and vulnerability to HIV and HCV in young Aboriginal people, which will be critically important for addressing epidemics in this population.Footnote 27 Dr. Stephen Moses and colleagues demonstrated that male circumcision can significantly reduce the spread of HIV.Footnote 28 Dr. Eric Cohen is contributing significantly to the understanding of HIV pathogenesis and has uncovered the mechanism through which a specific HIV protein (HIV-1 Vpu accessory) affects Tetherin, a host restriction factor that potently blocks HIV-1 release and viral transmission.Footnote 29
Antibiotic resistance
Canadian researchers are also contributing to world literature in the field of antibiotic resistance. Figure 3 shows that Canada ranks third for relative citations, suggesting that Canadian publications on antibiotic resistance and infection control are of high quality and have high impact. The data also show that Canada ranks third for relative change in number of publications from 1997-2008, with a 4.9-fold increase for a total of 1,258 publications - an indication of a growing community.
The number of publications is represented by the size of the circles. A specialization index (SI) greater than 1.0 means that a particular country is more specialized in a certain area when compared to the world average and an average of relative citations (ARC) of greater than 1.0 indicates that a paper or a group of papers is cited more than the world average. Publications on antibiotic resistance were identified through Medical Subject Headings (MeSH searches done by the Observatoire des Sciences et des Technologies. Databases searched may not cover all publications in this area and ARC data are incomplete for 2008. Countries were ranked based on total number of publications (2000-2008).
Figure 3: Specialization index and average of relative citations for top 10 countries publishing in antibiotic resistance and infection control, 2000-2008
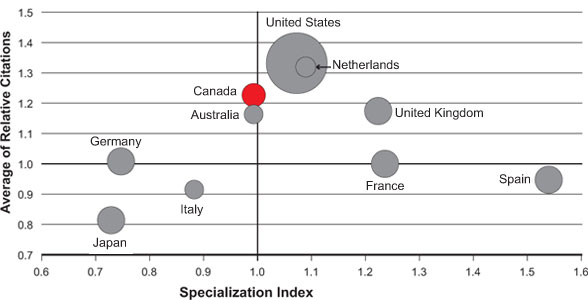
III anticipates that its recent initiatives on antibiotic resistance will result in an increased publication baseline as the community becomes more established and forges new international linkages through III's Canada-UK partnership on antibiotic resistance. Already, one of the team leads funded under the Novel Alternatives to Antibiotics request for applications (RFA), Alan Davidson at the University of Toronto, reports that: "The RFA on Novel Alternatives to Antibiotic Resistance allowed my group to move into a totally new and exciting area that is highly relevant to human disease. We would have had great difficulty in obtaining funds for this new research through regular funding channels because our proposed research was novel and not previously undertaken in my lab. Our RFA-funded project has already produced unexpected and very provocative results related to the effect of phages on the pathogenicity of Pseudomonas aeruginosa, and the potential use of phage-derived therapies against this important human pathogen."
Capacity building
The Institute has addressed capacity building through the CIHR Strategic Training Initiative in Health Research (STIHR), financially supporting 13 training programs relevant to the III mandate program, for a financial commitment of $9.4 million. These programs have supported graduate students, postdoctoral fellows and health care practitioners from a variety of fields and disciplines. Based on progress and end-of-grant reports, trainees from these programs have gone on to receive additional scholarships from federal and provincial agencies, and many are now academic researchers, postdoctoral fellows or tenure-track faculty. Others have chosen careers in industry, as clinical fellows or in science management. In the words of one of the STIHR team leaders:
"A significant achievement of our CIHR STIHR program over the past seven years is that it has brought together 16 laboratories from three Montréal-area universities working on neuroinflammation, including clinicians working in the two large multiple sclerosis clinics located at McGill University and Université de Montréal, as well as others working on neuroinflammation in spinal cord injury and maternal infections and brain development. The regular group activities made possible by this training program have tremendously enhanced the quality of the training we now offer our students and fellows."
In addition, the Institute has hosted three young investigator meetings and funded Pilot Project Grants targeted to new investigators. Overall, III has invested 29% of its strategic budget in capacity-building programs (47% in 2003-2004). Also, the Institute provides annual one-year bridge funding for III-related projects that narrowly missed the funding cut-off in the CIHR Open Operating Grant Program. Almost half of these grants have gone to young investigators, most of whom went on to receive multi-year operating grant funding. Capacity building has also been integral to many of III's strategic research initiatives with a focus on team building, as described below.
Emerging threats
More than 80 trainees were supported by SARS funding and many national and international collaborations were created, including a research network established by Dr. Robert Anderson with hubs in Canada, Taiwan and Thailand for the study of emerging viral respiratory and hemorrhagic diseases. Prior to the creation of the PPSRI, only 17 projects related to influenza were funded by CIHR in open competitions - a number that has now grown to 103. PPSRI has funded 10 team grants as well as international grants, including the China-Canada collaboration. The PHAC-CIHR Influenza Research Network (PCIRN) alone now includes more than 100 researchers.
The CIHR HIV/AIDS Research Initiative
The CIHR HIV/AIDS Research Initiative has focused on building future research capacity across all disciplines, and since 2000 has supported 194 individual training and salary awards and one of two STIHR grants relating to HIV/AIDS. This support has built considerable net HIV/AIDS research capacity including an increase in the number of biomedical investigators receiving funding from the Initiative from 69 in 2001-2002 to 141 in 2008-2009, and an increase in the overall CIHR investment in HIV/AIDS research from $23.9 million in 2001-2002 to $41.7 million in 2009-2010.
The number of biomedical researchers receiving funding from the HIV/AIDS Research Initiative increased 104% between 2001 and 2009.
Antibiotic resistance
Phage therapy represents a potential adjunct or replacement for traditional antibiotics but, prior to the Novel Alternatives to Antibiotics Initiative, CIHR did not fund research in this area. The initiative funded four projects, including three team grants - a funding commitment of $4.7 million - which is an impressive example of capacity building in an underserved research area.
Informing decision making
Emerging threats
Safe food and water
One objective of this initiative was to engage decision makers in the research process. As an example of success, a multidisciplinary team focused on safe drinking water and led by Dr. Isaac-Renton, overcame disciplinary and jurisdictional boundaries to focus on community health. The study engaged experts in each area along the source-to-tap continuum. As a result, provincial decision makers used research results to alert local residents of the need to get their well water tested regularly for E. coli contamination.Footnote 30
In another example, Dr. Mazumder and his team persuaded farmers to reduce the time their cattle spent in rivers in order to reduce bacterial contamination of river water. In one case, river contamination was reduced by building a bridge across the river which re-routed cattle away from the water. Researchers also distributed an information pamphlet prompting individual farmers to contact researchers. As a result, the farmers became full partners in the subsequent research projects. Successes such as these may not lead to publication in top-tier journals, but they demonstrate the added value of III's strategic initiatives, which have achieved outcomes that would not have been realized in open competition funding. Meanwhile, the results of this research have had almost immediate impacts on population health.
SARS
The CIHR response to SARS produced numerous valuable research results as the organization dealt with the immediate health crisis and managed subsequent outbreaks such as H1N1. III coordinated a multi-sector response to SARS and created the SARS Research Consortium to improve communications among the research and public health communities. Information gained from a study of the long-term psychological consequences of the SARS outbreak has been widely communicated and incorporated into pandemic planning in Canada and Australia, and by the World Health Organization (WHO).Footnote 31
Pandemic influenza
Many research contributions to decision making during the H1N1 pandemic originated from research begun during the SARS outbreak. Examples include the evaluation of existing vaccination policies, patient prioritization strategies for treatment, and the strategic use of anti-virals by the Pandemic Influenza Outbreak Research Modeling Team, headed by Dr. Seyed Moghadas.Footnote 32,Footnote 33 Their results were incorporated into the Canadian vaccination policy developed during the H1N1 pandemic.Footnote 34 The team is now addressing significant public health concerns for protecting vulnerable populations from emerging infectious diseases, and seeking funds to address the relevant objectives. Also, the Canadian Program of Research on Ethics in a Pandemic (CanPREP), led by Dr. Ross Upshur, has made significant progress in informing policy and decision makers of the ethical challenges when they respond to a pandemic.Footnote 35 The College of Physicians and Surgeons in both Ontario and Nova Scotia have adopted recommendations proposed by CanPREP.
The CIHR HIV/AIDS Research Initiative
A Focus on Ethics
The Canadian Association for HIV Research, with support from III, the CIHR Ethics Office and other partners, developed and launched the 2008 document Ethics Issues for Canadian HIV/AIDS Researchers in International Settings.Footnote 36 This resource has been widely distributed nationally and internationally and provides background information and practical examples to help Canadian researchers through the ethical issues of HIV/AIDS research in international settings. It also responds to an identified need to sensitize our own HIV/AIDS researchers and policy makers so that they understand the ethical tensions of North-South HIV/AIDS research and policy projects.
Health and health system/care impacts
It often takes many years for research outcomes to impact health care and the health system. Change is rarely the result of a single study or even initiative. Impacts in this area, therefore, have arisen primarily from early III initiatives.
Emerging threats
SARS and influenza
Much of the knowledge and experience gained during the SARS outbreak proved invaluable during subsequent outbreaks, including research related to the ethics of outbreak management and control. For example, Dr. Ross Upshur used his experience during the SARS outbreak to develop an ethical framework for pandemic influenza. The framework was adapted by WHO and has been incorporated into pandemic plans in Canada, the U.S., New Zealand and Europe. Similarly, Drs. Eleanor Fish and James Dennis, who also received III SARS funding, devised a new treatment protocol for SARS infection that has been adopted by WHO and various jurisdictions around the globe, including the U.S. and Canada. Having demonstrated the therapeutic benefits of treating an acute viral respiratory infection with IFN alfacon-1,Footnote 37 Dr. Fish has gone on to investigate the potential for this treatment protocol in other viral respiratory infections, most notably influenza A virus infections.Footnote 38 Her studies have led to a randomized clinical trial in place for the upcoming flu season in Ontario. Specifically, patients hospitalized with severe influenza-like illness will be randomized for treatment with Tamiflu plus IFN alfacon-1 or Tamiflu plus placebo.
The CIHR HIV/AIDS Research Initiative
The CIHR Canadian HIV Trials Network (CTN) has a 20-year history of leading Canadian involvement in HIV clinical trials by providing the required infrastructure and expertise to conduct world-class trials. CTN and its nationally affiliated investigators continue to contribute to improved clinical management of HIV and better quality of life for those infected. As an example, CTN led the Options with Antiretrovirals trial, jointly funded by CIHR, the UK Medical Research Council and U.S. Veterans Affairs, which investigated various treatment strategies for people with advanced HIV who had failed on multiple treatment regimes.Footnote 39 The study has contributed important evidence on the safety of treatment interruptions that did not compromise patient safety in this population. In the words of Dr. Jonathan Angel, at the Ottawa Health Research Institute and a Core Leader of CTN: "Many HIV trials would have been impossible in Canada without the existence of the CTN and its transformative effect on clinical research. Not only has this led to the creation of new knowledge, it has improved the lives of people living with HIV and fostered the development of a new generation of clinical researchers."
Antibiotic resistance
One of the first teams III funded in the area of antibiotic resistance, led by Dr. Michael Mulvey, focused on antibiotic resistance in northern Canadian communities. As a result of III funding, and in partnership with the Public Health Agency of Canada, the Northern Antibiotic Resistance Partnership (NARP) was formed. NARP is comprised of community members, health care professionals, educators and researchers working together to study antibiotic-resistant bacteria, a major problem in northern communities where the rate far exceeds that in the rest of Canada. The group has developed the Germs Away teaching tool, aimed at grades 4 to 6, which teaches children how germs are spread and how to prevent it. This program has been piloted in 19 northern Saskatchewan schools and a decrease in community-associated methicillin resistant staphylococcus aureus (CA-MRSA) is already evident.Footnote 40 The program is supported by a website,Footnote 41 which includes age-appropriate hand washing posters, podcasts, training videos, a web-based video game, radio spots and guidelines for physicians on the management of CA-MRSA. The program is being rolled out in 150 schools in Nova Scotia - an excellent example of research in action with a demonstrated health outcome.
Economic impacts
The economic impacts of a microbial outbreak or pandemic can be severe, as was evident during the relatively brief period of the SARS outbreak. The Institute's ongoing efforts in the area of emerging threats are focused on better, more cost-effective treatments and preventive measures such as vaccines. Improved patient care will, in turn, lead to economic gains.
Attracting additional funds
Many of the research teams funded under III initiatives have gone on to leverage this targeted funding to expand the scope and breadth of their research and ensure sustainability. Some of these funds come from other CIHR programs but some are obtained from external sources as a direct result of initial funding provided by the Institute. Several examples follow.
Safe Food and Water Initiative
Dr. Subash Sad and his team went on to obtain further five-year funding in the Novel Alternatives to Antibiotic Initiative. Dr. Judith Isaac-Renton reports that her team has continued to work on other projects and has obtained additional research funding largely because of the network that CIHR spawned through its initial support. Dr. Neil Cashman reports that the grant he received in the Safe Food and Water Initiative facilitated the funding of a new Network of Centres of Excellence (NCE), PrioNet.
The CIHR HIV/AIDS Research Initiative
An excellent example of the significant economic impact of well-constructed, community-based research is the Positive Spaces, Healthy Places (PSHP) study. The first longitudinal community-based initiative in Canada, PSHP, led by Dr. Sean Rourke, is examining the relationship between housing status, housing stability and the factors that influence housing; and health outcomes for people living with HIV. The PSHP partnership helped Fife House, a housing service provider in Toronto, secure $19 million in government funding to increase the supply of supportive housing for people with HIV. The partnership also helped AIDS Niagara obtain an additional $200,000 in annual funding for supportive housing through the Local Health Integration Network.
Antibiotic resistance
Dr. Hans Vogel reports a major impact on his research area as a result of an III grant in the Novel Alternatives to Antibiotics Initiative, which has enabled his team to obtain additional funding from the Alberta Heritage Foundation for Medical Research for The Alberta Sepsis Network, providing a pipeline to clinical work and creating more employment opportunities for researchers and trainees.
Commercialization - the development of new products and technologies
Several projects funded under III strategic initiatives have produced new products and technologies. For example, a non-typhoidal Salmonella vaccine developed by Drs. Subash Sad and Brett Finlay led to additional funding from the CIHR Proof of Principle Program and Dr. Finlay is now in discussions with a potential industry partner to bring the vaccine to market. The III response to SARS resulted in one spin-off company, three patent applications and sufficient additional funding to create 30 jobs.Footnote 6
The Novel Alternatives to Antibiotics initiative, after only two years of funding, has produced early economic outcomes. Dr. Jonathan Dennis already has a patent pending on the technology related to phage aerosolization, which could make phage therapy a viable treatment option for persistent lung infections, including bacterial infections common in cystic fibrosis patients.Footnote 42 Another early outcome reported from this same initiative, by the team led by Dr. Gerry Wright, is the discovery of new targets for novel therapies to increase the potency of existing antibiotics.Footnote 43 This work has resulted in collaboration with a French biotechnology firm, Mutabilis, to pursue the drug discovery potential of the pathway. Yet another researcher supported through this initiative, Dr. David Heinrichs, reports that his research has resulted in three patent applications and an additional $600,000 in funding from a company to continue studies on the potential of staphylococcal proteins as vaccine antigens.
Transformative effects of the Institute
The Institute has exerted its transformative effects by bringing together researchers and research users across disciplines and sectors and galvanizing the III research community to respond to challenges and opportunities in under-researched and emerging areas. The initiatives described in this report would not have been possible, at least on the same scale, had the Institute not remained flexible and focused in its ability to respond rapidly to unanticipated health threats. As documented in the 2006 CIHR International Review and numerous impact assessments III has commissioned on its initiatives and on overall Institute achievements, it is clear that III has successfully delivered on its mandate. Much of this success is due to the tireless efforts and dedication of III's inaugural scientific director, Dr. Bhagi Singh, and his extraordinary leadership skills. It is fitting that his pivotal role in establishing III is soon to be honoured with the Bhagirath Singh Award for the III young researcher with the highest-ranking grant each year in CIHR's open competition.
Emerging threats
Safe food and water
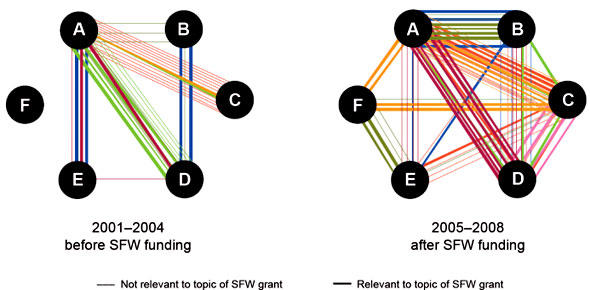
The Safe Food and Water Initiative brought together for the first time academic and government researchers to address a health issue of relevance to five government departments. Many collaborations created during this initiative have proven sustainable, with researchers continuing to collaborate and publish together (Figure 4) even after the funding ended.Footnote 20
III's Safe Food and Water Initiative created sustainable collaborations between government and academic researchers.
Each colour represents a different team. Each circle represents one member of a funded team. Some teams had six members (A-F), while others had four (A-D) or five (A-E). A line linking team members represents a co-publication.
Figure 4: Co-publications among members of funded teams in the Safe Food and Water Initiative (SFW)
SARS and pandemic influenza
III's rapid and coordinated response to SARS paved the way for the pandemic preparedness initiative. Pandemic Preparedness Strategic Research Initiative (PPSRI) task group member, Dr. Earl Brown, said: "The PPSRI has transformed the Canadian research base to a dynamic force, a trained cohort of individuals that embody the 'one health' concept of human and animal disease that can act in a meaningful way to improve our readiness and ability to respond to infectious disease threats as they arise in our globally connected nation."
In addition, the PHAC-CIHR Influenza Research Network (PCIRN), a group of more than 100 Canadian researchers, was created to assess vaccine safety, immunogenicity and effectiveness, as well as program implementation and evaluation. By November 2010, PCIRN had expanded to include over 30 Canadian universities, hospital and institutions, in six provinces.
The CIHR HIV/AIDS Research Initiative
In the HIV/AIDS field, the III approach has been truly transformative, bringing disparate groups together around a common agenda. The launch of the Centres for Population Health and Health Services Research Development in HIV/AIDS created a new way of supporting HIV health services and population health research and is expected to mobilize the research talent and increase the productivity and impact of research in these areas. The program provides infrastructure support for the Centres and facilitates partnership building and knowledge translation. After only one year of funding, both funded Centres have established national networks of researchers and knowledge users, established governance structures and priorities and are ready to undertake new, collaborative and strategic HIV research.
Going Forward
Going forward, III will continue to monitor the outcomes of previously funded initiatives. An understanding of the impact of prior strategic investments will guide future planning in all areas of the III mandate, including the development of the Institute's third strategic plan, for 2013-2018. New strategic priorities will align with CIHR's second strategic plan, called Health Research Roadmap: Creating innovative research for better health and health care. The five overarching research priority areas identified in this plan are listed here, followed by corresponding examples of current and planned III initiatives:
- Enhance patient-oriented care and improve clinical results through scientific and technological innovations - clinical autoimmunity, antibiotic resistance, HIV/AIDS, pandemic preparedness
- Support a high-quality, accessible and sustainable health care system - pandemic preparedness, HIV/AIDS, infection control, transplantation
- Reduce health inequities of Aboriginal peoples and other vulnerable populations - antibiotic resistance, HIV/AIDS
- Prepare for and respond to existing and emerging threats to health - pandemic preparedness, antibiotic resistance; vector-borne disease
- Promote health and reduce the burden of chronic disease and mental illness - clinical autoimmunity, human microbiome, inflammation
Lessons learned
The Institute has learned the importance of careful strategic planning to identify areas in which modest strategic investment can have an impact. The Institute has also learned the benefits of capacity building and the value of collaborations and partnerships in setting research agendas and leveraging resources. The lessons learned by managing the research response to numerous health threats since 2000 has taught III the importance of strong, proactive leadership - a tradition that will be upheld by the Institute's second scientific director.
Emerging threats - An ongoing challenge
New health threats constantly appear on the horizon. Food and waterborne illness is still commonplace and, although extreme public health measures curtailed the damage done by SARS, and the H1N1 pandemic was relatively mild, the world still lives in fear of the next pandemic. Globalization and climate change exert an influence on disease transmission and the vectors that carry disease, and new pathogens sporadically cross the species barrier, posing a new threat to human health. Despite these challenges, Canada is much better prepared than ever before to face health threats. Through III leadership, a strong foundation has been built to respond rapidly and effectively to threats to human health. For example, the range of expertise represented in the PHAC-CIHR Influenza Research Network extends far beyond influenza. Network investigators have designed it to respond to a broad range of public health threats in which vaccines would play a role.
Building on success
Pandemic influenza
A midterm evaluation of the Pandemic Preparedness Strategic Research Initiative (PPSRI) ascertained that the initiative's design, delivery and initial outputs ensure that the goals identified at the outset will be achieved.Footnote 8 As funding for PPSRI draws to a close, the program's overall success will be determined by an evaluation of the end-of-grant reports and the translatability and overall benefit of the research. The evaluation's outcome will inform future strategic initiatives addressing biological threats.
HIV/AIDS
Areas currently the focus of strategic initiative development within the CIHR HIV/AIDS Research Initiative are: addressing the complex issues of co-morbidities faced by people living with HIV/AIDS; HIV vaccine research; and further refinement of the HIV/AIDS Community-based Research Program. Given the strong alignment of priorities within the HIV/AIDS Initiative and Roadmap, many exciting opportunities are expected to arise that will be mutually beneficial for meeting the needs of the HIV and other research communities.
Antibiotic resistance
The Institute has launched several initiatives to support the antibiotic resistance community, including a partnership with the UK Medical Research Council (UK-MRC). In 2009, catalyst grants were awarded to two teams of Canadian and UK researchers and in September 2010, III and the UK-MRC launched a joint call for team grants. Successful teams will be supported equally by III and the UK-MRC (£2 million and $4 million) for four years, and will bring together the best researchers in both countries to advance antibiotic resistance research and provide new treatments and interventions.
New opportunities
Although III will remain vigilant, maintaining the Institute's reputation for innovative, rapid and flexible action when confronted by unexpected health crises, the Institute plans to launch new strategic initiatives.
Microbes: the world within us
The number of microbial cells associated with the human body outnumbers host cells by a factor of at least 10:1 and they encode approximately 100-fold more genetic information than the human genome. These microbes play an essential role in human health. Disruptions in the normal microbial flora can lead to disease and may be associated with a number of chronic health conditions. Due to the proactive actions of III's founding scientific director, Dr. Bhagi Singh, Canada is already an established member of the International Human Microbiome Initiative through the creation of the Canadian Microbiome Initiative (CMI). CMI has funded 12 catalyst grants and seven team grants, for a total investment by III and partners of $16.6 million. This initiative is integral to the international effort and places Canada third for financial contributions. Going forward, III will work with the newly funded teams to establish collaborations and international linkages that will ensure Canada maintains its leadership position in this international effort.
Inflammation: the silent killer
Inflammation is a tightly regulated process and dysfunction contributes to a plethora of disorders such as asthma, autoimmune diseases, atherosclerosis, obesity, inflammatory bowel diseases, transplant rejection and cancer. Persistent, low grade inflammation is now thought to be a common cause of many chronic diseases. The Institute is co-leading a multi-institute initiative that will focus on strategic research areas within this otherwise relatively well-funded research area. Already, III has identified transplantation as one potential focus. With an acute organ shortage in Canada, a donation rate that is half the rate of countries such as France and Spain and a high rejection rate caused primarily by chronic inflammation, transplantation poses some interesting questions beyond biomedical and clinical concerns.
Supporting the III community
The Institute will continue to support the III research community by listening to its concerns and responding where possible. The research community will be informed of III activities and funding opportunities as they arise, and III will be present at national meetings for information exchange and consultation. The practice of using bridge funding to support III researchers competing in CIHR open competitions will continue, and III will consult widely with the IAB and community to develop new strategies and initiatives. III will also continue the tradition of hosting young investigators meetings and conferences to sustain the outstanding cadre of researchers currently engaged in infection and immunity research. The new III team in Québec and Ottawa, under the leadership of Dr. Marc Ouellette, is committed to building on progress made during the Institute's first 10 years and looks forward to a successful and productive future.
List of Acronyms and Abbreviations
CIHR Institutes
| IAPH | Institute of Aboriginal Peoples' Health |
| IA | Institute of Aging |
| ICR | Institute of Cancer Research |
| ICRH | Institute of Circulatory and Respiratory Health |
| IGH | Institute of Gender and Health |
| IG | Institute of Genetics |
| IHSPR | Institute of Health Services and Policy Research |
| IHDCYH | Institute of Human Development, Child and Youth Health |
| III | Institute of Infection and Immunity |
| IMHA | Institute of Musculoskeletal Health and Arthritis |
| INMHA | Institute of Neurosciences, Mental Health and Addiction |
| INMD | Institute of Nutrition, Metabolism and Diabetes |
| IPPH | Institute of Population and Public Health |
III specific
| ARC | average of relative citations |
| BSE | Bovine spongiform encephalopathy |
| CA-MRSA | community-associated methicillin resistant staphylococcus aureus |
| CanPREP | Canadian Program of Research on Ethics in a Pandemic |
| CBR | community-based research |
| CHARAC | CIHR HIV/AIDS Research Advisory Committee |
| CHVI | Canadian HIV Vaccine Initiative |
| CMI | Canadian Microbiome Initiative |
| CTN | CIHR Canadian HIV Trials Network |
| H1N1 | porcine influenza |
| H5N1 | avian influenza |
| IAB | Institute Advisory Board |
| ISTC | International Science and Technology Centre |
| KT | knowledge translation |
| MeSH | U.S. National Library of Medicine Medical Subject Headings |
| NARP | Northern Antibiotic Resistance Partnership |
| NCE | Networks of Centres of Excellence |
| OPTIMA | Options with Antiretrovirals |
| PCIRN | PHAC-CIHR Influenza Research Network |
| PHAC | Public Health Agency of Canada |
| PPSRI | Pandemic Preparedness Strategic Research Initiative |
| PSHP | Positive Spaces, Healthy Places |
| RFA | request for applications |
| SARS | severe acute respiratory syndrome |
| SFW | safe food and water |
| SI | specialization index |
| STIHR | Strategic Training Initiative in Health Research |
| UKMRC | United Kingdom Medical Research Council |
| WHO | World Health Organization |
- Date modified: