Ethics Performance Measurement Annual Report 2015–2016
Table of Contents
- Introduction
- 1. Results Highlights
- 2. Advancing knowledge: CIHR ethics leadership strengthened at a national level
- 3. Informing decision-making: Strengthened accountability for ethics within CIHR
- 4. Building capacity: Strengthened and expanded national ethics knowledge base
- 5. Building capacity: Strengthened ethics research community in Canada
- 6. Indicators for which data will be available in future
- Conclusion
- Appendix 1: Summary of Standing Committee on Ethics (SCE) Issues and CIHR Action Taken, September 2014 – March 2016
- Appendix 2: Ethics-related Grants and Awards Validation Exercise
- Appendix 3: Operational Definitions for Ethics-related Grants and Awards
- Appendix 4: Detailed Results for Ethics-related Grants and Awards by Program
- Appendix 5: Details on Indicators for which Data will be Available in Future
Introduction
This is the first annual report prepared by CIHR, framed by the CIHR Ethics Action Plan, and detailing the results of the performance metrics set out in the Ethics Performance Measurement Strategy (PM Strategy), as endorsed by the CIHR Standing Committee on Ethics.
This report represents an important milestone for ethics at CIHR, providing baseline data for comparisons over time with respect to CIHR’s performance in meeting its commitments. As set out in the Ethics Action Plan, CIHR has committed to:
- Strengthen ethics leadership and impact in meeting CIHR’s mandate; and
- Systematically embed ethics, consideration of ethical issues, and application of ethical principles into CIHR’s business.
Implementation of the Ethics PM Strategy has necessitated putting in place new processes and methods for systematic collection of data. In some cases, particularly for long-term impacts of CIHR’s investments in grants and awards related to ethics, the data will come on stream in the future.
Efforts were made to keep performance indicators (and associated data collection requirements) in the Ethics PM Strategy to a manageable level while still ensuring key performance elements were covered. A consequence of this streamlined approach is that a process to systematically collect data on activities of CIHR Institutes with respect to national and international leadership in ethics was not put in place in time for reporting in 2015–2016. This could be another area for expansion of data collection and reporting in future years.
1. Results Highlights
The Ethics PM Strategy outlines the intended transformative outcomes set out for Ethics at CIHR and sets out indicators for measuring performance against these outcomes. Highlights of the results available for 2015–2016 are reported under key outcome areas.
| Key Outcomes | Measuring Performance: Results Highlights for 2015–2016 |
|---|---|
| Advancing Knowledge: CIHR ethics leadership strengthened at a national level |
|
| Informing Decision-making: Strengthened accountability for ethics within CIHR |
|
| Building Capacity: Strengthened and expanded national ethics knowledge base |
|
| Building Capacity: Strengthened ethics research community in Canada |
|
2. Advancing knowledge: CIHR ethics leadership strengthened at a national level
2.1 Indicators
- Number of education sessions/webinars/publications
- Number of participants in ethics education events
- Percentage satisfaction rate of participants after education session
- Number of hits on ethics education webpages
- Number of external presentations, meetings (national and international), and public products related to ethics at CIHR
2.2 Results
a) Education sessions/webinars/publications:
- CIHR continues to promote the CIHR Ethics Office Education Workbook as a useful educational tool, particularly for new investigators and trainees. Four education sessions using the Education Workbook were delivered with involvement of the CIHR Ethics Office in 2015–2016.
b)- c) Number of participants in ethics education events and satisfaction rate
- Due to ethics education sessions being delivered either remotely by the Ethics Office, or by others at the request of the Ethics Office, attendance and evaluation were not collected.
d) Number of hits on ethics education webpages (in both official languages unless reported separately):
- Number of sessions (visits to pages within a given time frame) on the ethics education website: 921 (0.07% of total sessions on CIHR web site)
- Number of page views: 2,563 (0.08% of total CIHR page views)
- Average time on page: 2:23 minutes (English); 2:25 minutes (French)
- Downloads of the Workbook, Ethics in research: Science lifecycle approach (PDF): 81
- Clicks on two Ethics Education YouTube Videos (English only): 12 unique events.
e) External presentations and public products:
- CIHR staff or representatives presented at two national meetings related to ethics (not including ethics education sessions).
- Three documents were published in 2015–2016: the Ethics Action Plan, the CIHR Chief Scientific Officer's Communiqué, and the Ethics Performance Measurement Strategy.
- Extensive updates of ethics content on CIHR's website were undertaken in 2015 and in 2016.
2.3 Detailed Results
2.3.1 Ethics Education
The CIHR Ethics Office Education Workbook consists of a Knowledge-to-Action/Ethics framework, and a series of scenarios where an ethics lens is applied and discussed. Plans are to develop new case studies for the Workbook in 2016–2017. Ethics education sessions using the Education Workbook are listed in the table.
| Ethics Education Sessions with | Date | Audiences | Description |
|---|---|---|---|
| Alberta Innovates | Nov 2015 Dec 2015 |
Employees | Ethics Education: Two separate sessions were delivered by the Ethics Office via video-conference. Covered: the Education Workbook with one case on social media and one on transgender issues. Remote delivery: attendance and evaluation not collected. |
| Canadian Association of Research Ethics Boards (CAREB) National Conference | May 2015 | CAREB members | Ethics Education: Delivered by a researcher at the request of the Ethics Office. Covered: the Education Framework and one case study on social media developed by the Ethics Office (different from the one mentioned above). Attendance and evaluation not collected. |
| Canadian GE3LS and Health Services & Policy Research Conference (CIHR Institute of Genetics) | April 2015 | Trainees | Ethics Education: Delivered by a researcher at the request of the Ethics Office. Covered: the Education Workbook and one case study on genomics and GE3LS. Attendance and evaluation not collected. |
CIHR hosted two education events with guest speakers as part of the Canadian Bioethics Society’s National Health Ethics Week 2015, which were held in March 2015 and therefore fall outside of the 2015–2016 scope of this report. There were 33 staff in total who participated in these sessions, and responses on the post-session evaluation surveys indicated high satisfaction levels. CIHR’s two education sessions as part of the National Health Ethics Week 2016 were held in April 2016, and will be reported in the Annual Report 2016–2017.
2.3.2 Ethics Education Webpages
During 2015–2016, the ethics education content on the CIHR website in both official languages was reviewed over the course of 921 sessions (i.e., visits to pages within the CIHR website within a given timeframe). While this represents a small proportion (0.07%) of the total sessions on the CIHR website, this is nonetheless particularly successful because the visits were deliberate (i.e., the average length of time spent on a page was over two minutes).
Most of these sessions originated in Canada (64%), the United States (8%), France (4%), and the United Kingdom (3%). However, some quality visits also originated in Cameroon (2%), Algeria (1%), French Guiana (<1%), Mali (<1%), and Madagascar (<1%). In addition, two YouTube videos of education sessions using the Workbook were viewed by 12 people in 2015–2016.
Comparing the traffic on the ethics education web pages to other web content on the CIHR site would not be informative, as this content has a specific objective and reach that is not similar to other CIHR projects. These web metrics will serve as a baseline for comparison going forward; however, we may consider establishing targets on a forward-looking basis (e.g., monitoring trends in traffic as they relate to events).
2.3.3 External presentations at National Meetings and Public Products
External presentations (not including ethics education sessions reported in section 2.3.1) and public products in 2015–2016 are listed in the following table.
| External Presentations at National Meetings | Date | Audiences | Description |
|---|---|---|---|
| Canadian Association of Research Ethics Boards (CAREB) National Conference | May 2015 | CAREB members | CIHR Update: Delivered for CIHR by the Executive Director of the Secretariat on Responsible Conduct of Research |
| Canadian Bioethics Society (CBS) Annual Conference | May 2015 | CBS members | CIHR Update: Presented by the CIHR Chief Scientific Officer (CSO). Covered: approval by Governing Council of the SCE’s Terms of Reference; and SCE’s achievements since 2014. |
| Public Products | Date | Audiences | Description |
|---|---|---|---|
| Web updates | September 2015 | All stakeholders, public | Updated Ethics @ CIHR content, Ethics Reform content, Responsible Conduct of Research content, Learning content, News content. |
| March 2016 | All stakeholders, public | Improvements to landing page (e.g., rotating banner), updated events page, updated contact information page (linking to the Government Electronic Directory Service). |
| External Presentations at National Meetings | Date | Audiences | Description |
|---|---|---|---|
| Ethics Action Plan | September 2015 | CIHR, health ethics community | Posted on website. Promotion was delayed due to the federal election period. Document was subsequently promoted in conjunction with the Performance Measurement Strategy, via the CIHR CSO Communiqué, and featured on the CIHR Ethics landing page |
| Communiqué from the CIHR CSO | March 2016 | All stakeholders, public | Sent by email to: SCE, CBS, CAREB, and CIHR Scientific Directors. Posted on the CIHR website, featured on: CIHR home page, Twitter, Monthly e-bulletin. |
| Ethics Performance Measurement Strategy | March 2016 | CIHR, health ethics community | Posted on website, promoted via Communiqué from the CIHR CSO, and featured on ethics landing page. |
In addition:
- A CIHR Scientific Director who is a member of the SCE and represents CIHR as a member of the Global Alliance for Genomics and Health (GA4GH) communicated SCE feedback on draft GA4GH policies which were open for public consultation, to the relevant GA4GH Working Group in June 2015.
- The Ethics Office issued an e-bulletin of CIHR’s ethics-related research funding opportunities on an approximate monthly basis, targeted to the health ethics community and, in particular, ethics researchers.
3. Informing decision-making: Strengthened accountability for ethics within CIHR
3.1 Indicators
- Percentage of CIHR governance & advisory committees that include ethics stakeholders
- Number of issues addressed by SCE that become action items for CIHR
- Number of CIHR policies containing an ethics component
- Ratio of internal committees and working groups where ethics is present (such as the Partnerships Risk Working Group) and not present
3.2 Results
a) Governance and Advisory Bodies:
- 90% of governance bodies (9 out of 10) included ethics stakeholders.
- 56% of CIHR-led advisory bodies (15 out of 27) included ethics stakeholders.
b) SCE Issues becoming CIHR Action Items:
- 15 out of 16 issues addressed by the SCE became action items for CIHR during the period September 2014 (when the SCE was established with a renewed mandate and membership) to March 2016.
c) CIHR Policies:
- 9 out of 12 CIHR policies in effect in 2015–2016 and applicable to CIHR-funded research had an ethics component.
d) Internal Committees and Working Groups:
- 90% of CIHR internal committees and working groups (53 out of 59) that were active in 2015–2016, had ethics representation. This constitutes a 9 to 1 ratio of CIHR internal committees and working groups where ethics was present and not present.
3.3 Definitions
Advisory bodies are defined as CIHR-led bodies active in 2015–2016 which provide advice to CIHR, do not have decision-making authority, and are not part of the formal governance structure of CIHR.
Ethics being present on internal committees and working groups is defined as having a member who is one of the following:
- Staff with ethics as a corporate responsibility at CIHR, as evident in the title of their position or direct corporate reporting.
- Staff who are expected to bring an ethics perspective.
- Staff from the Science, Knowledge, Translation and Ethics Branch, who are expected to represent the Branch, and provide liaison to the CIHR Ethics Office for ethics-related matters as needed.
Ethics component of a policy is defined as content that explicitly refers to ethics or ethical concepts, or compliance with ethical policies.
Ethics stakeholders on governance and advisory bodies are defined as one of the following:
- Staff with ethics as a corporate responsibility at CIHR, as evident in the title of their position or direct corporate reporting.
- Individuals who are expected to bring an ethics perspective, and who may be staff or external individuals.
Governance bodies are defined as bodies operational in 2015–2016 that form part of the governance structure of CIHR for decision-making purposes. Subcommittees of decision-making bodies were included as "governance bodies", since these subcommittees form part of the official governance structure of CIHR with respect to their role of bringing recommendations to the governance body for decision.
Policy is defined as:
- A formal science-related Policy, Statement or Guide that sets out mandatory conditions or expectations applicable to CIHR-funded research. These policies may originate from CIHR, the Tri-Agencies (CIHR, NSERC and SSHRC) or the Federal Health Portfolio (of which CIHR is a part).
The following were not considered to be science-related "Policies": federal laws that apply to CIHR-funded research; CIHR strategic frameworks; CIHR education tools such as guides for applicants or peer reviewers; and internal CIHR policies governing such things as financial and human resource management or approval procedures.
3.4 Detailed Results
3.4.1 Governance
CIHR has 10 formal governance bodies (defined as bodies that form part of the governance structure of CIHR for decision-making purposes). These bodies are listed below, with ethics representation indicated where present.
| Governance bodies (2015–2016) | Ethics stakeholders on governance body |
|---|---|
| 1. Governing Council (GC) |
|
| 2. GC Executive Committee |
|
| 3. GC Audit Committee | -- |
| 4. GC Stem Cell Oversight Committee |
|
| 5. GC Standing Committee on Ethics |
|
| 6. GC Governance and Nominating Committee |
|
| 7. Executive Management Committee (EMC) |
|
| 8. Extended EMC |
|
| 9. Science Council (SC) |
|
| 10. SC Subcommittee on Implementation and Oversight |
|
3.4.2 Advisory Bodies
CIHR led or co-led 27 advisory bodies in 2015–2016, including the 13 Institute Advisory Boards. These bodies are listed in the following table, with ethics representation indicated where present.
| CIHR-led Advisory Body | Ethics stakeholders |
|---|---|
| 1. Canadian Clinical Trials Coordinating Centre Research Ethics Board Working Group on Accreditation |
|
| 2. CIHR HIV/AIDS Community-Based Research Steering Committee |
|
| 3. CIHR HIV/AIDS Research Advisory Committee | -- |
| 4. Drug Safety and Effectiveness Network Science Advisory Committee | -- |
| 5. Drug Safety and Effectiveness Network Steering Committee | -- |
| 6. CIHR Ethical, Legal and Social Issues (ELSI) Advisory Committee for the Canadian Longitudinal Study on Aging |
|
| 7. Ethics Advisory Committee on Innovative Clinical Trials |
|
| 8. Evidence Informed Health Care Renewal Working Group | -- |
| 9. National Steering Committee for Canada's Strategy for Patient-Oriented Research |
|
| 10. President's Advisory Committee on eHealth Innovation | -- |
| 11. Sub-committee on SPOR: External Data group on Data Strategy for SUPPORT Units |
|
| 12. Tri-Council (TC3) Data Management Plan Advisory Committee | -- |
| 13. Tri-Council Harmonization Project Advisory Group | -- |
| 14. Interim College of Reviewers Advisory Group | -- |
| Plus 13 Institute Advisory Boards (IABs)*
*These IABs were in operation through to April 2016. A new IAB system has been put in place as of 2016–2017. |
9 out of 13 IABs have :
|
3.4.3 SCE Issues that became Action Items for CIHR
The SCE held six face to face meetings during the period from September 2014 through March 2016, and convened via subgroup teleconferences as needed. A single broad issue may have been discussed at more than one meeting of the SCE, with different aspects highlighted for SCE attention, but was only counted once.
The 15 issues addressed by the SCE that resulted in CIHR actions are:
- Evaluation and data (a component of the CIHR Ethics Action Plan)
- Communication and engagement (a component of the CIHR Ethics Action Plan)
- Integration of ethics in CIHR (an component of the CIHR Ethics Action Plan)
- Global Alliance for Genomics and Health and associated policies
- Pan-Canadian Clinical Trial on Chronic Cerebrospinal Venous Insufficiency Procedure for Multiple Sclerosis
- Patient and citizen engagement in research
- Electronic consent for research
- Partnerships (the CIHR-wide partnership strategy, and partnerships with the private sector in the Pathways to Health Equity for Aboriginal Peoples Signature Initiative)
- Role of the SCE in the Canadian ethics landscape
- Disruptive Technologies
- Human germline gene editing
- Crowdfunding
- CIHR Strategic Action Plan on Training
- CIHR Health-related and Health Research Data Framework
- Global Health.
A detailed summary of the issues and resulting actions is provided in Appendix 1.
The issue "Innovative Methods for Clinical Trials" was brought to the SCE but CIHR considered that follow up action was not needed because of other work underway in Canada that overlapped in scope.
3.4.5 CIHR Policies containing an Ethics Component
Nine out of 12 CIHR and Tri-Council policies in effect in 2015–2016, and applicable to CIHR-funded research, contained an ethics component. These policies were:
- CIHR Grants and Awards Guide
- CIHR Policy Statement on Official Languages
- Tri-Agency Financial Administration Guide
- Tri-Agency Open Access Policy on Publications
- Tri-Agency Responsible Conduct of Research Framework
- Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans 2nd edition
- Agreement on the Administration of Agency Grants and Awards by Research Institutions
- Conflict of Interest and Confidentiality Policy of Federal Research Funding Organizations
- Health Portfolio Sex and Gender-Based Analysis Policy
The ethics content in these nine policies is summarized in the following table:
| CIHR and Tri-Council Policies | Summary of Ethics Component |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The three CIHR policies without explicit ethics components have very specific procedural objectives, as described in the following table.
| Policies not containing an ethics component | Policy Objectives |
|---|---|
| CIHR Policy Statement: Electronic Final Reports | Requires grantees to provide final reports to assist CIHR in evaluating its programs and measure the impacts of its funding. |
| Public Communications Policy of the Federal Research Funding Organizations | Requires Institutions and Agencies to collaborate to inform the public about Agency grants and awards, and impacts. |
| CIHR Policy on the Institutional Electronic Approval of Applications | Requires institutions to electronically approve applications submitted by Nominated Principal Investigators at their institutions. |
4. Building capacity: Strengthened and expanded national ethics knowledge base
4.1 Scope of Grants and Awards Data Collection for "Ethics"
The CIHR Standing Committee on Ethics (SCE) at its March 2016 meeting endorsed an expanded scope of grants and awards data collection for the Ethics PM Strategy to include grants and awards that relate directly to ethics, or indirectly to ethics (i.e., law and a subset of socio-cultural factors affecting health), to be reported separately.
Figure 1. Scope of Grants and Awards Data Collection: Directly and Indirectly Related to Ethics
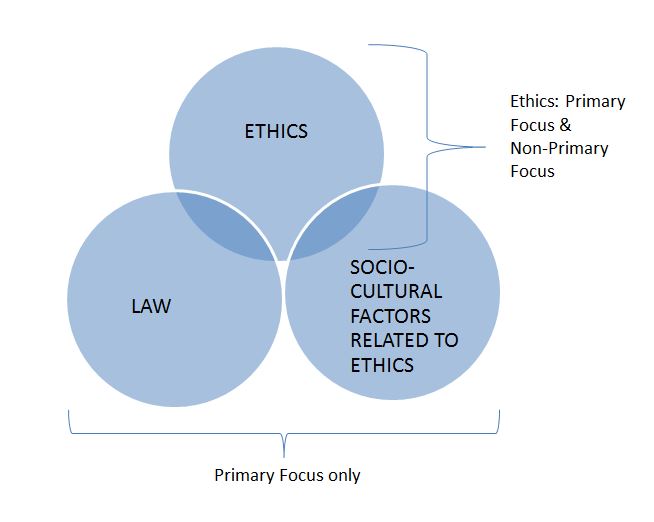
As illustrated in Figure 1, grants and awards with ethics as a primary focus, and as a non-primary focus (i.e., where ethics is a component of a grant or award but not the main focus), were included in the analysis, since ethics is the main focus of the Ethics PM Strategy. Grants and awards exploring law or relevant socio-cultural factors were included only as a primary focus, thus minimizing the counting of grants and awards in more than one category. However, it was possible for grants and awards to fit into the category of Ethics Non-Primary Focus, as well as Law-Primary Focus or Socio-Cultural Primary Focus (the overlapping areas in Figure 1).
Operational definitions for ethics, law, and social-cultural factors related to ethics, were also endorsed, with an expectation that the CIHR Ethics Office would further refine the inclusion and exclusion criteria for the socio-cultural category to minimize overlap with standard CIHR reports on investments related to the broad theme of "Social, Cultural, Environmental and Population Health Research". The Ethics Office's process for refining the inclusion and exclusion criteria and validating relevant grants and awards is described in Appendix 2. The operational definitions for each category endorsed by the SCE for the purposes of the Ethics PM Strategy, and further refined during the validation process, are provided in Appendix 3.
Ethics-related grants and awards for which funding had started in previous years and continued in 2015–2016, or funding was initiated in 2015–2016, were included in the analysis. These constitute all grants and awards "actively" funded in 2015–2016. In addition, a separate analysis was conducted that included only those grants and awards for which funding started in 2015–2016.
Given the newly broadened scope for grants and awards data collection to include all those directly or indirectly related to ethics, the results reported in 2015–2016 will constitute a baseline for future comparisons over time.
4.2 Indicators
- Percentage of total CIHR expenditures for ethics research (i.e., ethics-related grants and awards)
- Number of ethics grants and awards in the investigator-initiated research and priority-driven research opportunities
See Appendix 5-1(a) for details on indicators for which data will be available in future.
4.3 Results
These results constitute baseline data for grants and awards funded in 2015–2016, to be used for comparisons over time. “Actively funded” refers to grants and awards for which funding started or continued in 2015–2016. “New” grants and awards had funding start in 2015–2016.
Double-counting was minimal: for all actively funded grants and awards, three “Ethics Non-Primary Focus” grants/awards were also counted in the Socio-cultural category (of which two had funding start in 2015–2016).
a) Percentage of total CIHR expenditures for ethics-related grants and awards in 2015–2016:
| Scope | Actively funded G & A in 2015-2016(New and Continuing) | New G & A in 2015-2016 |
|---|---|---|
| % of CIHR Total ($973 million) | % of CIHR Total ($226 million) | |
| Note. “G & A” refers to grants and awards. Expenditures for “Ethics as a Non-Primary Focus” cannot be reported because there are no data on amounts for grants/awards budgets allocated to ethics components. | ||
| Ethics-Primary Focus | 0.37% ($3.58 million) | 0.32% ($0.72 million) |
| Law-Primary Focus | 0.06% ($0.56 million) | 0.11% ($0.25 million) |
| Socio-Cultural Factors related to Ethics-Primary Focus | 1.66% ($16.17 million) | 2.16% ($4.90 million) |
b) Number of ethics-related grants and awards in the investigator-initiated research and priority-driven research opportunities
Figure 2. Number of Actively Funded (New and Continuing) Ethics-related Grants and Awards in 2015–2016, and Percentage of CIHR Total Grants and Awards, by Funding Type.
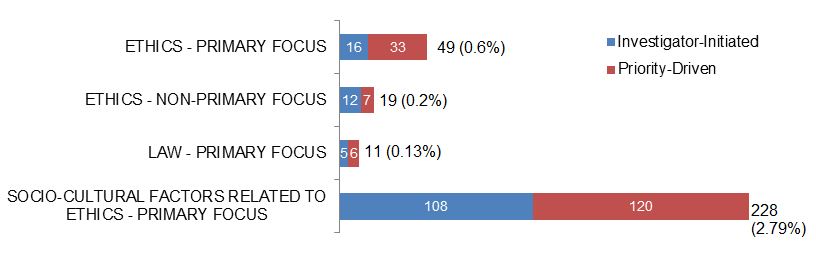
Figure 2 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Ethics - Primary Focus | 16 | 33 |
| Ethics - Non-Primary Focus | 12 | 7 |
| Law - Primary Focus | 5 | 6 |
| Socio-Cultural Factors Related To Ethics - Primary Focus | 108 | 120 |
Note. CIHR funded a total of 8201 grants and awards (new and continuing) in 2015–2016.
Figure 3. Number of New Ethics-related Grants and Awards in 2015–2016, and % of CIHR Total New Grants and Awards, by Funding Type.
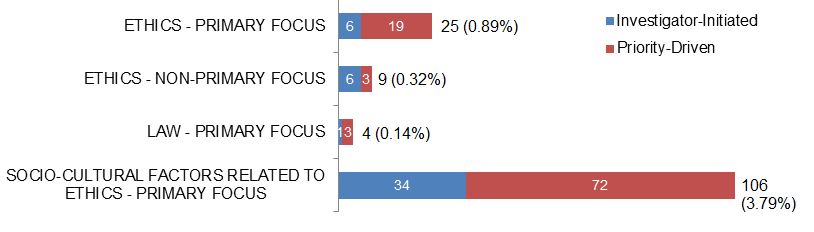
Figure 3 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Ethics - Primary Focus | 6 | 19 |
| Ethics - Non-Primary Focus | 6 | 3 |
| Law - Primary Focus | 1 | 3 |
| Socio-Cultural Factors Related To Ethics - Primary Focus | 34 | 72 |
Note. CIHR funded a total of 2798 new grants and awards in 2015–2016.
Detailed results for Ethics-related Grants and Awards by Program (research operating grants, training and career support awards, etc.) are provided in Appendix 4.
4.4 Definitions
Awards are funded through Investigator-initiated and Priority-driven sources, and refer to funding to researchers and trainees to support training (Master's, PhD, Postdoctoral Fellow) or career advancement (Chairs, salary awards). Travel awards and prizes are also included in this category.
Grants are funded through Investigator-initiated and Priority-driven sources, and include support for the direct costs of research projects; and support for conferences and workshops to establish research priorities; researcher networking and collaborative activities; scientific exchanges between Canadian and international researchers; programs that inform researchers and other stakeholders about aspects of health research; and grants to selected organizations engaged in research-related activities such as the Canadian Council on Animal Care.
Investigator-Initiated funding type (also known as "curiosity-driven" or "open" funding) refers to funding through competitions open to any area of health research.
Priority-Driven funding type (also known as "strategic" funding) refers to funding through initiatives by CIHR and its Institutes to address a specific area of need. Priority-driven research includes grants and awards funded through Signature and Strategic Initiatives, CIHR Institute funding opportunities, Catalyst Grants in specific areas of research such as ethics, and "Priority Announcements" (PAs) on open competitions (which offer additional sources of funding for highly rated applications that are relevant to specific CIHR research priority areas or mandates).
5. Building capacity: Strengthened ethics research community in Canada
5.1 Indicators
- Number of funding opportunities of Institutes and Initiatives for which an ethics perspective was offered at the design stage
- Number of funding opportunities of Institutes and Initiatives for which an ethics perspective was offered and incorporated as appropriate
See Appendix 5-2 (a-f) for details on indicators for which data will be available in future.
5.2 Results
The results capture the efforts of the CIHR Ethics Office to provide an ethics perspective in the design of Priority-Driven funding opportunities.
a) Ethics perspective offered in development of targeted funding opportunities:
- The CIHR Ethics Office provided an ethics perspective in the design of 16 Priority-Driven funding opportunities launched in 2015–2016.
b) Ethics perspective incorporated in targeted funding opportunities:
- Ethics input offered by the CIHR Ethics Office was incorporated in 12 of the 16 funding opportunities for which advice was offered.
5.3 Definitions
Ethics content was defined as explicit reference in the funding opportunity to any of the following:
- Ethics considerations needing to be considered in the design of the research proposal and included in proposal evaluation criteria.
- Ethics being an eligible research area.
- Ethics expertise being appropriate for a research team.
- Ethics being included as a component of a proposed training program.
- Responsibility for “ethically sound” approaches to public engagement and partnerships.
The standard statement in funding opportunities that applicants must comply with ethical guidelines and Tri-Council policies was not counted as “ethics content” for the purposes of this indicator.
5.4 Detailed Results
A total of 73 Priority-Driven funding opportunities were launched in 2015–2016. Of these, 21 funding opportunities contained ethics content. The Ethics Office provided ethics content at some stage in the design of 12 of these 21 funding opportunities. Advice on ethics content in funding opportunities may come from a number of sources in addition to the Ethics Office, such as: other CIHR staff, including Institutes, with experience in ethics; members of the external community with ethics expertise; and Institute Advisory Board Ethics Designates. Also, the inclusion of a statement about the need to “consider ethical implications” of the proposed research, or similar wording, may be standard practice for some initiatives.
6. Indicators for which data will be available in future
Data were available for 13 of the 28 indicators in the Ethics PM Strategy. With respect to data collection for the remaining indicators:
- For five indicators that relate to numbers of funded ethics researchers, success rates, and workload of ethics peer reviewers, the Ethics Office anticipates reporting on 2015–2016 data in 2016-2017, after we have developed and validated a keyword methodology to query researchers, reviewers and applications relevant to the expanded scope of "ethics".
- Data collection for the remaining ten indicators is subject to: completion of funding decisions and releases for competitions launched in 2015–2016; completion of the upcoming recruitment of reviewers into the College of Reviewers; and availability of data on longer-term outputs of validated 2015–2016 grants and awards.
- At least partial data on longer-term outputs should be available starting in 2017-2018 from end-of-grant reports from recipients of validated grants and awards and bibliometric data.
More details are provided in Appendix 5.
Conclusion
This report on 2015–2016 activities describes how CIHR is meeting its commitments under the Ethics Action Plan, and provides baseline data to measure success over time.
In future years, a more complete picture of the middle and long term impacts of CIHR’s work in ethics will become evident as data collection is expanded and enhanced.
Appendix 1: Summary of Standing Committee on Ethics (SCE) Issues and CIHR Action Taken, September 2014 – March 2016
| Issue | Date issue was first submitted and by whom | Request(s) to SCE at one or more meetings on this topic | CIHR Actions | Status (as of July 2016) |
|---|---|---|---|---|
| 1. Evaluation and Data (Ethics Action Plan) | Sept 2014, CIHR Science, Knowledge Translation and Ethics (SKTE) Branch | Advise on the development of a Performance Measurement Strategy (PM Strategy) for Ethics, including how to capture the impact of ethics activities within open programs and strategic initiatives |
|
Ongoing |
| 2. Communication and Engagement (Ethics Action Plan) | Sept 2014, CIHR SKTE Branch | Advise on CIHR's communication strategy for ethics |
|
Ongoing |
| 3. Integration of Ethics (Ethics Action Plan) | Sept 2014, CIHR SKTE Branch | How to optimize the integration of ethics within CIHR (includes building research capacity in ethics, and education in ethics) |
|
Ongoing |
| 4. Global Alliance for Genomics and Health (GA4GH) | Sept 2014, CIHR President | Provide advice on the GA4GH Framework for Responsible Sharing of Genomics and Health-related Data, and related policies, and review against relevant Tri-Council Policies |
|
Completed |
| 5. Pan-Canadian Clinical Trial on Chronic Cerebrospinal Venous Insufficiency Procedure for Multiple Sclerosis | Sept 2014, CIHR President | Provide advice on the ethics of continuing or stopping the CIHR-co-funded study |
|
Completed |
| 6. Patient and Citizen Engagement | September 2014, CIHR Priority-Driven Research Branch | Provide feedback on SPOR’s Patient Engagement Framework and CIHR's Patient and Citizen Engagement Implementation Strategy, and associated tools; and an Ethics Office proposal to develop ethics guidance |
|
In development |
| 7. Partnerships with the private sector in Pathways to Health Equity for Aboriginal Peoples Signature Initiative, and the CIHR-wide Partnership Strategy | January 2015, CIHR-Institute of Population and Public Health and the CIHR Partnerships and Business Development Branch | Provide advice on 1) refining the partnership benefit-risk assessment approaches for the Pathways initiative, and 2) identifying key ethical considerations when developing a CIHR-wide partnership strategy |
|
Ongoing |
| 8. The role of SCE in the Canadian ethics landscape | January 2015, SCE | SCE identified lack of a national ethics committee in Canada, and question of scope of SCE's mandate |
|
Ongoing |
| 9. Ethics and Disruptive Technologies | May 2015, CIHR- Institute of Infection and Immunity and the SKTE Branch | Provide advice on ethics considerations in disruptive technologies |
|
Ongoing |
| 10. Electronic Consent | May 2015, SCE Member | To consider whether to bring the issue of electronic consent to the Panel on Research Ethics as an alternate means to document consent. |
|
Completed |
| 11. Human Germline Gene Editing | Sept 2015, CIHR Chief Scientific Officer | To advise on CIHR’s role |
|
In development |
| 12. Crowdfunding research | Sept 2015, CIHR Institute of Nutrition, Metabolism and Diabetes and the CIHR Institute of Population and Public Health | Provide advice on whether CIHR should permit or promote crowdfunding of research |
|
Ongoing |
| 13. CIHR Strategic Action Plan on Training | Sept 2015, CIHR SKTE Branch | Provide feedback on CIHR's draft Strategic Action Plan on Training |
|
Not yet initiated |
| 14. Global Health | Sept 2015, External SCE member and CIHR Ethics Office | Provide feedback on the Canadian Coalition for Global Health Research's (CCGHR) Draft Principles for Canadians Involved in Global Health Research. |
|
Completed |
| 15. CIHR Health-related and Health Research Data Framework | March 2015, CIHR SKTE Branch | Feedback on the integration of ethics in the Data Framework's objectives and proposed action plan. |
|
Completed |
| 16. Ethical Challenges arising from Innovative Methods for Clinical Trials | Jan 2015, CIHR Institute of Circulatory and Respiratory Health and the SKTE Branch | Have representation on an Advisory Group to be set up by CIHR to commission a white paper on innovative clinical trial methodology and guidance | CIHR decided not to pursue this issue because of overlap with work underway by others in Canada. |
Appendix 2: Ethics-related Grants and Awards Validation Exercise
Data set: There are 8201 grants and awards (G & A) that actively received funds in the 2015- 2016 fiscal year. This includes G & A for which funding started prior to 2015-2016 and continued, or funding started in 2015-2016.All validation processes involved review of G & A titles, investigator-provided keywords, and Abstracts (if submitted).Foundation grants did not have Abstracts, and therefore grant Summaries (if submitted) were reviewed.
Definition of scope and categories: As described in section 4.1, the scope of G & A data collection was expanded to include G & A that relate directly to ethics, or indirectly to ethics (i.e., law and a subset of socio-cultural dimensions of health).Operational definitions of these categories were endorsed by the SCE, and are presented in Appendix 3.
Step 1: Initial development of inclusion/exclusion criteria for the "Socio-Cultural" (S-C) category
Ethics office staff extracted 202 G & A using a few keywords describing the socio-cultural bucket presented to SCE. Ethics Office staff came to a common understanding on an initial set of S-C inclusion/exclusion criteria.
Step 2: Further refinement of the S-C inclusion/exclusion criteria.
Using a 5% sample, Ethics Office staff reviewed the same 410 G & A for ethics primary and non-primary, law and socio-cultural focus; and discussed any differences in scoring.An interrater agreement of 95% was reached. Based on consensus, the S-C inclusion/exclusion criteria were further refined for Step 3.
Step 3: Validation of the remaining dataset, and further refinement of the Ethics-Primary and S-C inclusion/exclusion criteria.
After the validation exercise in Step 2, three Ethics Office staff validated the remaining 7791 G & A over a 2-3 week period.Validators scored: yes or no for "Ethics-primary focus", "Ethics-non-primary focus", and "Law" G & A; and indicated the particular inclusion criteria for any S-C G & A. Validators also indicated where they were unsure, and all of these G & A were discussed and scored by consensus.In discussion, inclusion criteria for the Ethics-Primary focus G & A and for the S-C category were refined.
Step 4: Analysis of the validated "Ethics" dataset
The Data & Statistics section of the Performance & Accountability Branch is responsible for doing the main analysis of the validated dataset.The Team Lead was sent the validated dataset with instructions on the analysis required as per the PM Strategy indicators.
Step 5: Sub-analyses
The Ethics office conducted sub-analyses after receiving the main analyses.
Step 6: Development of a keyword-based methodology
In subsequent years, the Ethics Office will develop a keyword methodology, using the validated 2015-2016 dataset, to report on indicators requiring the validation of large datasets of G & A (e.g., success rates in competitions) in order to make the workload manageable.To eliminate false positives, manual validation based on the operational definitions presented in Appendix 3 will continue to be an essential step of the validation process.
Additional Notes on the Validation Process:
Double-counting: Double-counting of G & A in more than one category was minimized as much as possible, although it was technically possible for projects to fit into the category of Ethics Non-Primary Focus, as well as Law-Primary Focus or Socio-Cultural-Primary Focus. In the 2015-2016 dataset, there were only three G & A that were double-counted due to being validated as both Ethics Non-Primary Focus and Socio-Cultural Primary Focus.
Unknowns: Of the 8201 G & A that actively received funds in 2015-2016, G & A were validated as being relevant to ethics or not.In addition, 1192 G & A had no Abstract or Summary available to the validators, and therefore could not be assessed.These G & A were therefore marked as "Unknown" and were not included in the validated dataset.Exceptions to this rule were made only if the G & A title explicitly indicated a central focus on ethics. The vast majority of "Unknown" projects were CIHR-funded Canada Research Chair Awards.
Appendix 3: Operational Definitions for Ethics-related Grants and Awards
1) Ethics as a Primary Focus and 2) Ethics as a Non-Primary Focus
Ethics-Operational definition:
The grant/award incorporates ethics as it applies to health (including health research, health promotion and maintenance, clinical care, population and public health, health systems and services, policy and governance). Relevant grants/awards are framed around, and expand upon, ethical principles, concepts or issues. Grants/awards may have ethics as a primary or non-primary focus. Both types will be captured, but will be reported separately.
Additional Inclusion criteria for Ethics as a Primary Focus:
- Ethics—either explicit use of the word “ethics/ethical” or of an ethics concept such as “informed consent”, “research integrity”, “conflict of interest”, “moral distress”—is the driver and central focus of the grant/award, even where there are other aspects to the grant/award (e.g., policy development).
- The grant/award has no abstract but the title clearly highlights a central focus on ethics.
- Grants funded through the Catalyst Grants-Ethics program.
3) Law as a Primary Focus
Law-Operational definition:
The grant/award explores law-related aspects of health and health research as a primary focus beyond compliance with existing legal frameworks. Grants/awards may or may not have an explicit ethical dimension to the project.
4) Socio-Cultural Factors related to Ethics as a Primary Focus:
Socio-Cultural Operational definition:
The grant/award explores social and cultural dimensions of health and health research as a primary focus. These grants/awards may or may not explicitly reference ethics or involve ethics researchers. Projects explore concepts related to ethics such as:
- cultural appropriateness and norms;
- vulnerability, marginalization and stigmatization;
- loss or devaluation of language and culture;
- access to culturally appropriate materials and services;
- equity/inequity.
Additional Inclusion Criteria:
1. A primary focus on:
- exploring (and developing) theories or frameworks of ethics-related concepts and principles
- exploring Aboriginal cultural ceremonies
- involving loss or devaluation of language and culture
- engaging stakeholders to elicit information on cultural appropriateness and norms (including gender norms and identities) -- e.g., in the design or development of an intervention. Exclude projects focused on implementation of an intervention- see 2b.
- engaging groups/individuals in vulnerable circumstances to find out what interventions, research priorities, etc., are appropriate for them
- exploring the causes (e.g., barriers) or consequences (e.g., impacts) of circumstances of vulnerability, marginalization or stigmatization on groups or individuals
- designing an intervention focused around ethics-related concepts (e.g., weight bias/stigmatization)
- implementing training focused on ethics-related concepts (e.g., culturally appropriate research methods/skills, cultural norms, vulnerability)
Exclusion Criteria:
2. A primary focus on:
- exploring causes of disease (including psychological conditions)
- implementing an intervention (e.g., health care delivery, training, education, networking) or disseminating the results of an intervention, even if targeted to persons or groups in circumstances of vulnerability. Training focused on ethics-related concepts falls under 1h and should be included.
- evaluating an intervention/program, even if the evaluation involves exploring the experiences and views of persons or groups in circumstances of vulnerability with regard to the intervention, unless the evaluation is directly informing a (re-)design of the intervention (see 1d).
- exploring a phenomenon/cause/event itself (e.g., climate change) , with a non-primary focus on the impact on, or experiences of, groups or persons in circumstances of vulnerability
- “vulnerability” as “risk of disease”, “risk of adverse effects of disease”, or “risk of unhealthy behaviours”
- engaging stakeholders, unless focused on 1d (cultural appropriateness/norms) or 1e (appropriateness for those in vulnerable circumstances)
Appendix 4: Detailed Results for Ethics-related Grants and Awards by Program
A4.1 Ethics-related Grants and Awards Actively Funded in 2015–2016 (New and Continuing)
A4.1a Ethics as a Primary Focus
Figure 4. Ethics as a Primary Focus: CIHR Expenditures (in Thousands) for New and Continuing Grants and Awards in 2015–2016, by Program Type.
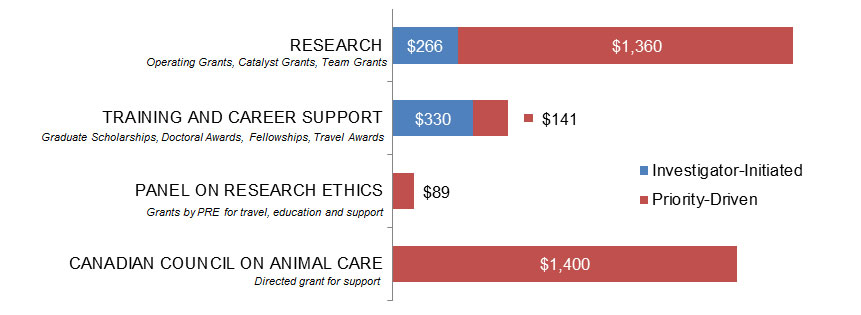
Figure 4 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | $266,274 | $1,359,767 |
| Training And Career Support | $329,500 | $141,250 |
| Panel On Research Ethics | $88,600 | |
| Canadian Council On Animal Care | $1,400,000 |
Erratum: An error was corrected in the reported expenditures for the “Training and Career Support” category in Figure 4. Table 1 presents the correct data and remains unchanged.
Figure 5. Ethics as a Primary Focus: Number of New and Continuing Grants and Awards in 2015–2016, by Program Type.
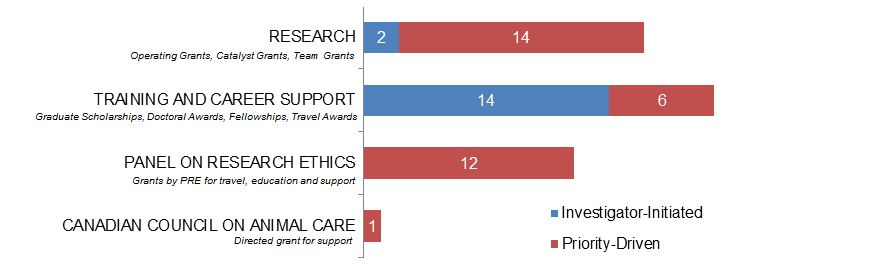
Figure 5 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 2 | 14 |
| Training And Career Support | 14 | 6 |
| Panel On Research Ethics | 12 | |
| Canadian Council On Animal Care | 1 |
| Programs | # | $ | % of $ |
|---|---|---|---|
| Investigator-Initiated | 16 | $595,774 | 16.62% |
| Research | 2 | $266,274 | 7.43% |
| Operating Grant | 2 | $266,274 | 7.43% |
| Training and Career Support | 14 | $329,500 | 9.19% |
| Canada Graduate Scholarships – Michael Smith Foreign Study Supplements | 2 | $12,000 | 0.33% |
| CIHR Fellowship | 6 | $157,500 | 4.39% |
| Doctoral Award - Doctoral Foreign Study Award (DFSA) | 1 | $10,000 | 0.28% |
| Doctoral Award - Frederick Banting and Charles Best Canada Graduate Scholarships | 4 | $116,667 | 3.25% |
| Doctoral: Vanier Canada Graduate Scholarships | 1 | $33,333 | 0.93% |
| Priority-Driven | 33 | $2,989,617 | 83.38% |
| Research | 14 | $1,359,767 | 37.92% |
| Catalyst Grant: Ethics | 8 | $680,868 | 18.99% |
| Catalyst Grant: HIV/AIDS Community-Based Research Program - General Stream | 1 | $32,715 | 0.91% |
| Operating Grant – Priority Announcement: HIV/AIDS Research Initiative - Health System/Population Health Stream | 1 | $76,439 | 2.13% |
| Operating Grant - Priority Announcement: Ethics | 2 | $209,545 | 5.84% |
| Operating Grant: Programmatic Grants to tackle health and health equity | 1 | $347,700 | 9.70% |
| Team Grant: European Research Projects of Neuroscience | 1 | $12,500 | 0.35% |
| Training and Career Support | 6 | $141,250 | 3.93% |
| Doctoral Research Award – Priority Announcement: Evidence Informed Healthcare Renewal | 1 | $2,500 | 0.07% |
| Doctoral Research Award - Priority Announcement: Research in First Nations, Métis &/or Inuit Health | 1 | $48,500 | 1.35% |
| Fellowship - Priority Announcement: Health Services/Population Health HIV/AIDS Research | 1 | $8,333 | 0.23% |
| Fellowship - Priority Announcement: Regional Partnership Program - Newfoundland & Labrador | 1 | $18,750 | 0.52% |
| Fellowship - Priority Announcement: Knowledge Translation | 1 | $61,667 | 1.72% |
| Travel Awards - Institute Community Support | 1 | $1,500 | 0.04% |
| Panel on Research Ethics | 12 | $88,600 | 2.47% |
| Canadian Council on Animal Care | 1 | $1,400,000 | 39.05% |
| Grand Total | 49 | $3,585,391 | 100.00% |
A4.1b Ethics as a Non-Primary Focus
Figure 6. Ethics as a Non-Primary Focus: Number of New and Continuing Grants and Awards in 2015–2016, by Program Type.
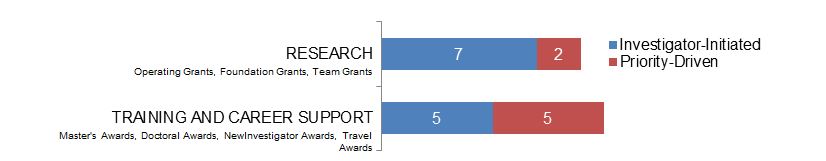
Figure 6 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 7 | 2 |
| Training And Career Support | 5 | 5 |
Note: Expenditures for “Ethics as a Non-Primary Focus” cannot be reported because there are no data on the amount of grant/award budgets allocated to ethics components.
| Programs | # |
|---|---|
|
*: Two of these operating grants were also counted in the Socio-Cultural category (Table 4). Note: Expenditures for “Ethics as a Non-Primary Focus” cannot be reported because there are no data on the amount of grant/award budgets allocated to ethics components. |
|
| Investigator-Initiated | 12 |
| Research | 7 |
| Foundation Grant | 2 |
| Operating Grant* | 5 |
| Training and Career Support | 5 |
| CIHR New Investigator | 1 |
| Doctoral Award: Frederick Banting and Charles Best Canada Graduate Scholarships | 3 |
| Master's Award: Frederick Banting and Charles Best Canada Graduate Scholarships | 1 |
| Priority-Driven | 7 |
| Research | 2 |
| Team Grant : Environments and Health - LOI - IWK/TEK/TES** | 1 |
| Team Grant: HIV/AIDS Vaccine Discovery and Social Research | 1 |
| Training and Career Support | 5 |
| Doctoral Award - Frederick Banting and Charles Best Canada Graduate Scholarships | 2 |
| Fellowship - Priority Announcement: Orphan Drug Policy | 1 |
| Travel Awards - Institute Community Support | 2 |
| Grand Total | 19 |
A4.1c Law as a Primary Focus
Figure 7. Law as a Primary Focus: CIHR Expenditures (in Thousands) by Program Type in 2015–2016.
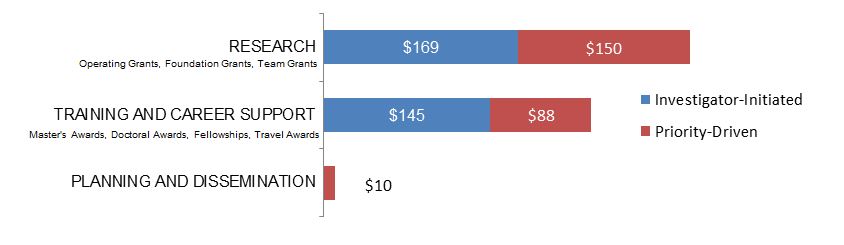
Figure 7 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | $169,008 | $149,967 |
| Training And Career Support | $145,000 | $87,959 |
| Planning And Dissemination | $10,000 |
Figure 8. Law as a Primary Focus: Number of New and Continuing Grants and Awards by Program Type in 2015–2016.
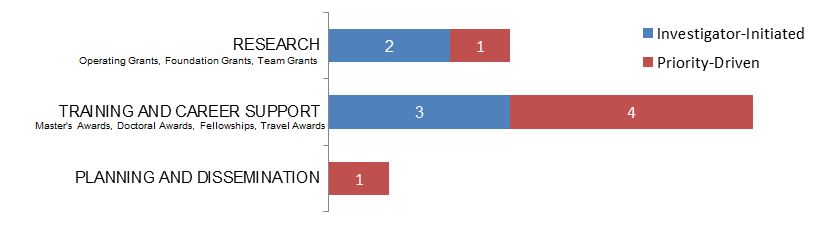
Figure 8 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 2 | 1 |
| Training And Career Support | 3 | 4 |
| Planning And Dissemination | 1 |
| Programs | # | $ | % of $ |
|---|---|---|---|
| Investigator-Initiated | 5 | $314,008 | 55.88% |
| Research | 2 | $169,008 | 30.08% |
| Operating Grant | 2 | $169,008 | 30.08% |
| Training and Career Support | 3 | $145,000 | 25.81% |
| CIHR Fellowship | 1 | $45,000 | 8.01% |
| Doctoral: Vanier Canada Graduate Scholarships | 2 | $100,000 | 17.80% |
| Priority-Driven | 6 | $247,926 | 44.12% |
| Research | 1 | $149,967 | 26.69% |
| Operating Grant - HIV/AIDS CBR Program - General | 1 | $149,967 | 26.69% |
| Training and Career Support | 4 | $87,959 | 15.65% |
| Doctoral Research Award: HIV/AIDS Community-Based Research Program - Aboriginal Stream | 1 | $37,500 | 6.67% |
| Doctoral Research Award: HIV/AIDS Community-Based Research Program - General Stream | 1 | $41,667 | 7.41% |
| Master's Award - HIV/AIDS Community-Based Research: General Stream | 1 | $7,292 | 1.30% |
| Travel Awards - Institute Community Support | 1 | $1,500 | 0.27% |
| Planning and Dissemination Grant | 1 | $10,000 | 1.78% |
| Planning and Dissemination Grant – Institute Community Support | 1 | $10,000 | 1.78% |
| Grand Total | 11 | $561,934 | 100.00% |
A4.1d Socio-Cultural Factors related to Ethics as a Primary Focus
Figure 9. Socio-Cultural Factors related to Ethics as a Primary Focus: CIHR Expenditures (in Thousands) for New and Continuing Grants and Award in 2015–2016, by Program Type.
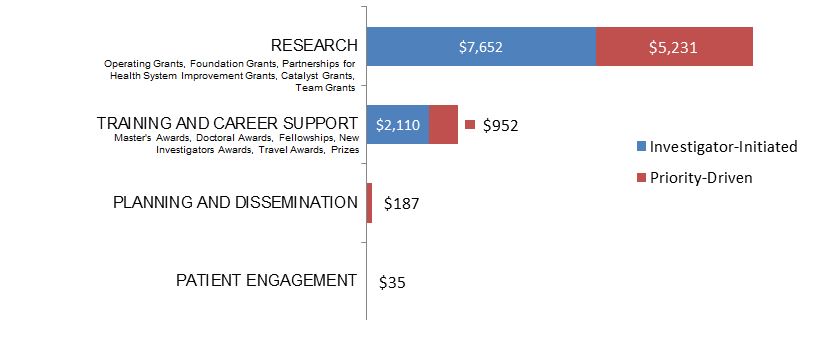
Figure 9 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | $7,652,081 | $5,231,275 |
| Training And Career Support | $2,109,999 | $952,173 |
| Planning And Dissemination | $186,683 | |
| Patient Engagement | $35,000 |
Figure 10. Socio-Cultural Factors related to Ethics as a Primary Focus: Number of New and Continuing Grants and Awards in 2015–2016. by Program Type.
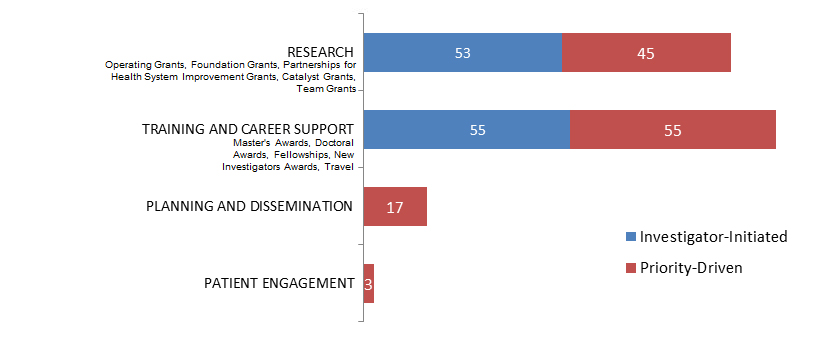
Figure 10 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 53 | 45 |
| Training And Career Support | 55 | 55 |
| Planning And Dissemination | 17 | |
| Patient Engagement | 3 |
| Programs | # | $ | % of $ |
|---|---|---|---|
*: Two of these operating grants were also counted in the Ethics as a Non-Primary Focus category (Table 2). |
|||
| Investigator-Initiated | 108 | $9,762,080 | 60.38% |
| Research | 53 | $7,652,081 | 47.34% |
| Foundation Grant | 1 | $1,078,088 | 6.67% |
| Operating Grant* | 50 | $6,321,477 | 39.10% |
| Operating Grant: Knowledge to Action | 1 | $117,255 | 0.73% |
| Partnerships for Health System Improvement | 1 | $135,261 | 0.84% |
| Training and Career Support | 55 | $2,109,999 | 13.05% |
| Banting Postdoctoral Fellowship Program | 1 | $140,000 | 0.87% |
| CIHR Fellowship | 9 | $329,999 | 2.04% |
| CIHR New Investigator | 8 | $420,000 | 2.60% |
| Doctoral Award - Doctoral Foreign Study Award | 1 | $27,500 | 0.17% |
| Doctoral Award - Frederick Banting and Charles Best Canada Graduate Scholarships | 16 | $495,000 | 3.06% |
| Doctoral: Vanier Canada Graduate Scholarships | 9 | $450,000 | 2.78% |
| Master's Award: Frederick Banting and Charles Best Canada Graduate Scholarships | 9 | $157,500 | 0.97% |
| New Investigator Salary Award - Priority Announcement: Knowledge Translation | 1 | $60,000 | 0.37% |
| Peter Lougheed/CIHR New Investigator Canada's Premier Young Researcher | 1 | $30,000 | 0.19% |
| Priority-Driven | 120 | $6,405,131 | 39.62% |
| Research | 45 | $5,231,275 | 32.37% |
| CANADA-HOPE Scholarship Program - Operating Grant | 2 | $2,709 | 0.02% |
| Catalyst Grant: HIV/AIDS Community-Based Research Program - General Stream | 2 | $66,000 | 0.41% |
| Catalyst Grant: HIV/AIDS Community Based Research Program - Aboriginal Stream | 4 | $131,663 | 0.81% |
| Collaborative Health Research Projects (NSERC partnered) | 1 | $55,500 | 0.34% |
| Emerging Team Grant: Alliances in Mobility in Aging - full application | 2 | $500,000 | 3.09% |
| HIV Implementation Science – Component 1 | 1 | $150,000 | 0.93% |
| Operating Grant - Priority Announcement: Applying the "Two-eyed Seeing" model to Aboriginal Health | 3 | $400,000 | 2.47% |
| Operating Grant - HIV/AIDS Community-Based Research Program - Aboriginal | 3 | $396,466 | 2.45% |
| Operating Grant - HIV/AIDS Community-Based Research Program - General | 4 | $581,951 | 3.60% |
| Operating Grant - Priority Announcement: Aboriginal Ways of Knowing and Two-eyed Seeing (Bridge) | 3 | $300,000 | 1.86% |
| Operating Grant - Priority Announcement: Canadian HIV Vaccine Initiative Vaccine Discovery and Social Research | 1 | $127,218 | 0.79% |
| Operating Grant - Priority Announcement: Regional Partnership Program - Nova Scotia | 1 | $17,429 | 0.11% |
| Operating Grant - Priority Announcement: Regional Partnership Program - Saskatchewan | 1 | $2,450 | 0.02% |
| Operating Grant - Priority Announcement: Regional Partnership Program : Manitoba | 1 | $17,263 | 0.11% |
| Operating Grant - Priority Announcement: Aboriginal Ways of Knowing | 2 | $301,521 | 1.87% |
| Operating Grant: Autism Spectrum Disorders Treatment and Care Research | 1 | $40,000 | 0.25% |
| Operating Grant: Cancer Prevention Research Grants | 1 | $149,974 | 0.93% |
| Operating Grant: Health, Wellbeing & Extended Working Life | 1 | $31,250 | 0.19% |
| Operating Grant: Population Health Intervention Research | 2 | $257,007 | 1.59% |
| Operating Grant: Programmatic Grants to tackle health and health equity | 1 | $400,000 | 2.47% |
| Operating Grant: SPOR PIHCI Network: Quick Strikes | 1 | $95,000 | 0.59% |
| Team Grant: Environments and Health – Letter of Intent (LOI) - Indigenous Ways of Knowing (IWK) / Traditional Ecological Knowledge (TEK) / Two-Eyed Seeing (TES) Nexus Areas * | 1 | $49,789 | 0.31% |
| Team Grant: Environments and Health – Letter of Intent (LOI) - Resource development | 1 | $50,000 | 0.31% |
| Team Grant: Community-Based Primary Healthcare - Full Application | 1 | $439,756 | 2.72% |
| Team Grant: Community-Based Primary Healthcare – Institute of Aboriginal Peoples Health (IAPH) | 1 | $500,000 | 3.09% |
| Team Grant: Pathways Implementation Research Team – Component 2 | 3 | $168,329 | 1.04% |
| Training and Career Support | 55 | $952,173 | 5.89% |
| Doctoral Research Award - Priority Announcement: Aboriginal Research Methodologies | 6 | $191,500 | 1.18% |
| Doctoral Research Award - Priority Announcement: Health Services/Population Health HIV/AIDS Research | 2 | $68,500 | 0.42% |
| Doctoral Research Award - Priority Announcement: IAPH Quantitative Research | 1 | $38,500 | 0.24% |
| Doctoral Research Award - Priority Announcement: Regional Partnerships Program - Saskatchewan | 1 | $17,500 | 0.11% |
| Doctoral Research Award - Priority Announcement: Patient-Oriented Research | 1 | $30,000 | 0.19% |
| Doctoral Research Award - Priority Announcement: Research in First Nations, Métis &/or Inuit Health | 3 | $94,500 | 0.58% |
| Doctoral Research Award: HIV/AIDS CBR Program - General Stream | 1 | $2,500 | 0.02% |
| Fellowship - PA: Health Services/Population Health HIV/AIDS Research | 3 | $89,167 | 0.55% |
| Fellowship - PA: Mixed Methods Approach in Aboriginal Research | 1 | $30,000 | 0.19% |
| Fellowship - PA: Research in First Nations, Métis and/or Inuit Health | 1 | $23,334 | 0.14% |
| Fellowship - Priority Announcement: Aboriginal Research Methodologies | 1 | $45,583 | 0.28% |
| Fellowship - Priority Announcement: IAPH Quantitative Research | 2 | $48,334 | 0.30% |
| New Investigator Award - PA: HIV/AIDS Services/Population Health Research | 2 | $135,000 | 0.84% |
| New Investigator Salary Award – Priority Announcement: Knowledge Translation | 1 | $45,000 | 0.28% |
| Prize – Institute of Human Development Child and Youth Health (IHDCYH) Talks | 1 | $750 | 0.00% |
| Training Grant: Indigenous Mentorship Network Program – Letter of Intent (LOI) | 2 | $49,497 | 0.31% |
| Travel Awards - Institute Community Support | 26 | $42,508 | 0.26% |
| Planning and Dissemination | 17 | $186,683 | 1.16% |
| Commonwealth Fund Dissemination Award 2015–2016 | 1 | $2,500 | 0.02% |
| Planning and Dissemination Grant– Institute Community Support | 16 | $184,183 | 1.14% |
| Patient Engagement | 3 | $35,000 | 0.22% |
| Patient Engagement – Collaboration Grants | 3 | $35,000 | 0.22% |
| Grand Total | 228 | $16,167,211 | 100.00% |
A4.2 New Ethics-related Grants and Awards in 2015–2016
A4.2a Ethics as a Primary Focus
Figure 11. Ethics as a Primary Focus: Expenditures (in Thousands) for New Grants and Awards in 2015–2016, by Program Type.
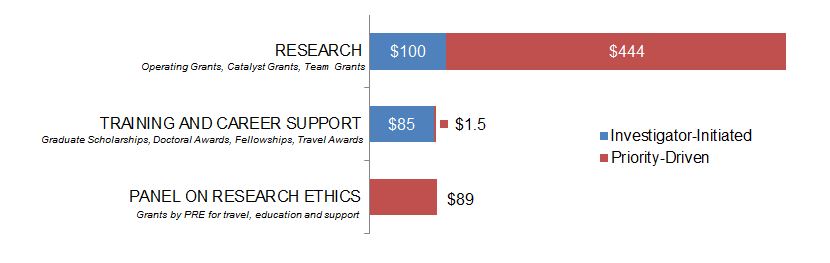
Figure 11 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | $100,000 | $443,986 |
| Training And Career Support | $84,500 | $1,500 |
| Panel On Research Ethics | $88,600 |
Figure 12. Ethics as a Primary Focus: Number of New Grants and Awards in 2015–2016, by Program Type.
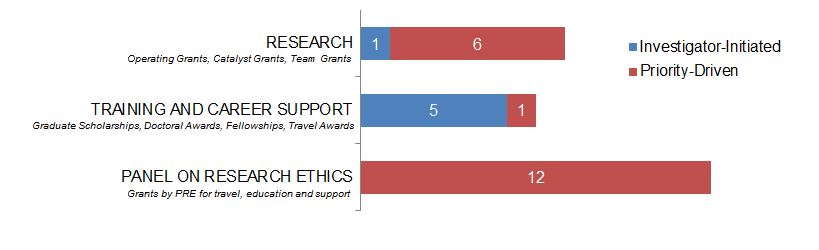
Figure 12 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 1 | 6 |
| Training And Career Support | 5 | 1 |
| Panel On Research Ethics | 12 |
| Programs | # | $ | % of $ |
|---|---|---|---|
| Investigator-Initiated | 6 | $184,500 | 25.68% |
| Research | 1 | $100,000 | 13.92% |
| Operating Grant | 1 | $100,000 | 13.92% |
| Training and Career Support | 5 | $84,500 | 11.76% |
| Canada Graduate Scholarships – Michael Smith Foreign Study Supplements | 2 | $12,000 | 1.67% |
| CIHR Fellowship | 2 | $37,500 | 5.22% |
| Doctoral Award - Frederick Banting and Charles Best Canada Graduate Scholarships | 1 | $35,000 | 4.87% |
| Priority-Driven | 19 | $534,086 | 74.32% |
| Research | 6 | $443,986 | 61.79% |
| Catalyst Grant: Ethics | 4 | $398,771 | 55.49% |
| Catalyst Grant: HIV/AIDS CBR Program - General Stream | 1 | $32,715 | 4.55% |
| Team Grant: European Research Projects of Neuroscience | 1 | $12,500 | 1.74% |
| Training and Career Support | 1 | $1,500 | 0.21% |
| Travel Awards - Institute Community Support | 1 | $1,500 | 0.21% |
| Panel on Research Ethics | 12 | $88,600 | 12.33% |
| Grand Total | 25 | $718,586 | 100.00% |
A4.1b Ethics as a Non-Primary Focus
Figure 13. Ethics as a Non-Primary Focus: Number of New Grants and Awards in 2015–2016, by Program Type.
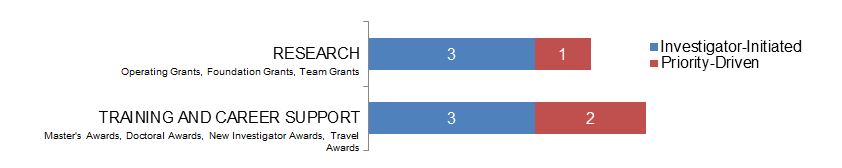
Figure 13 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 3 | 1 |
| Training And Career Support | 3 | 2 |
Note: Expenditures for “Ethics as a Non-Primary Focus” cannot be reported because there are no data on the amount of grant/award budgets allocated to ethics components.
| Programs | # |
|---|---|
*: This grant is also counted in the Socio-Cultural category (Table 8). Note: Expenditures for “Ethics as a Non-Primary Focus” cannot be reported because there are no data on the amount of grant/award budgets allocated to ethics components. |
|
| Investigator-Initiated | 6 |
| Research | 3 |
| Foundation Grant | 2 |
| Operating Grant* | 1 |
| Training and Career Support | 3 |
| CIHR New Investigator | 1 |
| Doctoral Award: Frederick Banting and Charles Best Canada Graduate Scholarships | 1 |
| Master's Award: Frederick Banting and Charles Best Canada Graduate Scholarships | 1 |
| Priority-Driven | 3 |
| Research | 1 |
| Team Grant: Environments and Health - LOI - IWK/TEK/TES* | 1 |
| Training and Career Support | 2 |
| Travel Awards - Institute Community Support | 2 |
| Grand Total | 9 |
A4.1c Law as a Primary Focus
Figure 14. Law as a Primary Focus: Expenditures (in Thousands) for New Grants and Awards in 2015–2016, by Program Type
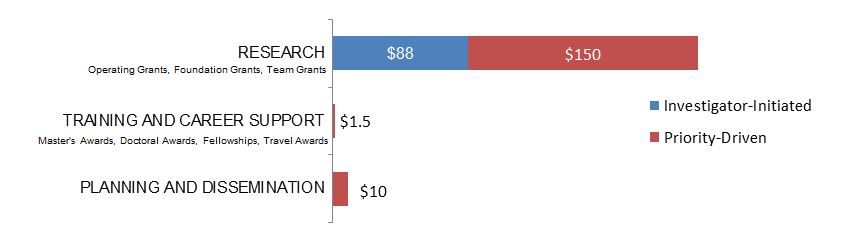
Figure 14 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | $88,496 | $149,967 |
| Training And Career Support | $1,500 | |
| Planning And Dissemination | $10,000 |
Figure 15. Law as a Primary Focus: Number of New Grants and Awards in 2015–2016, by Program Type.
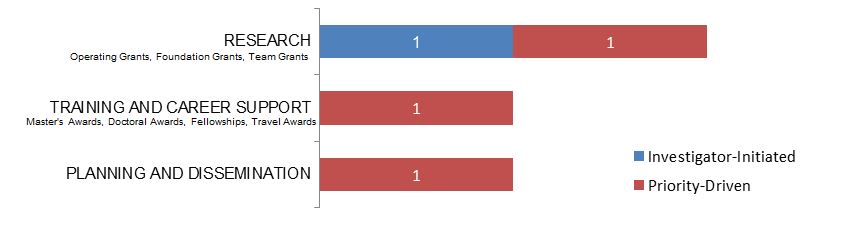
Figure 15 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 1 | 1 |
| Training And Career Support | 1 | |
| Planning And Dissemination | 1 |
| Programs | # | $ | % of $ |
|---|---|---|---|
| Investigator-Initiated | 1 | $88,496 | 35.40% |
| Research | 1 | $88,496 | 35.40% |
| Operating Grant | 1 | $88,496 | 35.40% |
| Priority-Driven | 3 | $161,467 | 64.60% |
| Research | 1 | $149,967 | 60.00% |
| Operating Grant - HIV/AIDS CBR Program - General | 1 | $149,967 | 60.00% |
| Training and Career Support | 1 | $1,500 | 0.60% |
| Travel Awards - Institute Community Support | 1 | $1,500 | 0.60% |
| Planning and Dissemination | 1 | $10,000 | 4.00% |
| Planning and Dissemination Grant – Institute Community Support | 1 | $10,000 | 4.00% |
| Grand Total | 4 | $249,963 | 100.00% |
4.1d Socio-Cultural Factors related to Ethics as a Primary Focus
Figure 16. Socio-Cultural Factors related to Ethics as a Primary Focus: Expenditures (in Thousands) for New Grants and Awards in 2015–2016, by Program Type.
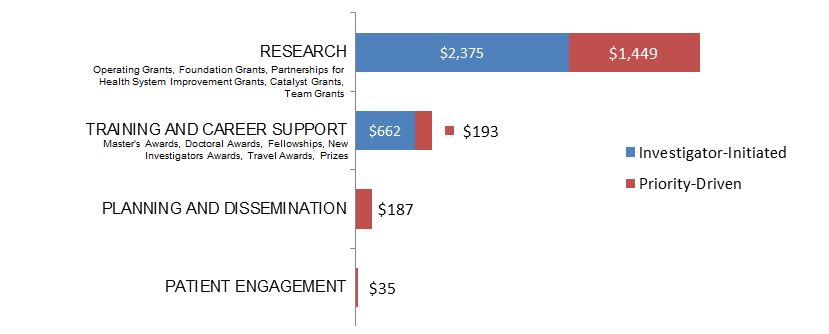
Figure 16 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | $2,374,903 | $1,449,012 |
| Training And Career Support | $661,666 | $193,255 |
| Planning And Dissemination | $186,683 | |
| Patient Engagement | $35,000 |
Figure 17. Socio-Cultural Factors related to Ethics as a Primary Focus: Number of New Grants and Awards in 2015–2016, by Program Type.
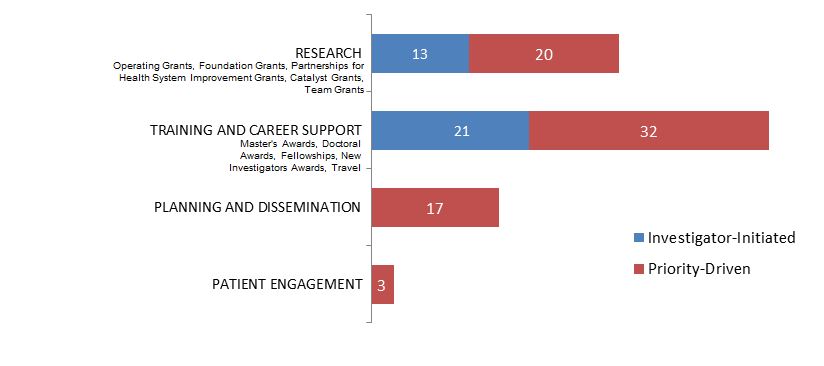
Figure 17 long description
| Investigator-Initiated | Priority-Driven | |
|---|---|---|
| Research | 13 | 20 |
| Training And Career Support | 21 | 32 |
| Planning And Dissemination | 17 | |
| Patient Engagement | 3 |
| Programs | # | $ | % of $ |
|---|---|---|---|
*One of these grants was also counted in the Ethics Non-Primary Focus category (Table 6). |
|||
| Investigator-Initiated | 34 | $3,036,569 | 61.96% |
| Research | 13 | $2,374,903 | 48.46% |
| Foundation Grant | 1 | $1,078,088 | 22.00% |
| Operating Grant* | 12 | $1,296,815 | 26.46% |
| Training and Career Support | 21 | $661,666 | 13.50% |
| Banting Postdoctoral Fellowship Program | 1 | $140,000 | 2.86% |
| CIHR Fellowship | 2 | $76,666 | 1.56% |
| CIHR New Investigator | 1 | $5,000 | 0.10% |
| Doctoral Award - Doctoral Foreign Study Award (DFSA) | 1 | $27,500 | 0.56% |
| Doctoral Award - Frederick Banting and Charles Best Canada Graduate Scholarships | 5 | $155,000 | 3.16% |
| Doctoral: Vanier Canada Graduate Scholarships | 2 | $100,000 | 2.04% |
| Master's Award: Frederick Banting and Charles Best Canada Graduate Scholarships | 9 | $157,500 | 3.21% |
| Priority-Driven | 72 | $1,863,950 | 38.04% |
| Research | 20 | $1,449,012 | 29.57% |
| HIV Implementation Science – Component 1 | 1 | $150,000 | 3.06% |
| Operating Grant - HIV/AIDS CBR Program - Aboriginal | 4 | $131,663 | 2.69% |
| Operating Grant - HIV/AIDS CBR Program - General | 2 | $66,000 | 1.35% |
| Operating Grant - Priority Announcement: Aboriginal Ways of Knowing and Two-eyed Seeing (Bridge) | 3 | $300,000 | 6.12% |
| Operating Grant: Cancer Prevention Research Grants | 1 | $149,974 | 3.06% |
| Operating Grant: Health, Wellbeing & Extended Working Life | 1 | $31,250 | 0.64% |
| Operating Grant: Population Health Intervention Research | 2 | $257,007 | 5.24% |
| Operating Grant: SPOR PIHCI Network: Quick Strikes | 1 | $95,000 | 1.94% |
| Team Grant: Environments and Health - LOI - IWK/TEK/TES** | 1 | $49,789 | 1.02% |
| Team Grant: Environments and Health - LOI - Resource development | 1 | $50,000 | 1.02% |
| Team Grant: Pathways Implementation Research Team – Component 2 | 3 | $168,329 | 3.43% |
| Training and Career Support | 32 | $193,255 | 3.94% |
| Doctoral Research Award - Priority Announcement: Aboriginal Research Methodologies | 1 | $33,500 | 0.68% |
| Doctoral Research Award - Priority Announcement: Research in First Nations, Métis &/or Inuit Health | 2 | $67,000 | 1.37% |
| Prize - IHDCYH Talks | 1 | $750 | 0.00% |
| Training Grant: Indigenous Mentorship Network Program - LOI | 2 | $49,497 | 1.01% |
| Travel Awards - Institute Community Support | 26 | $42,508 | 0.87% |
| Planning and Dissemination | 17 | $186,683 | 3.81% |
| Commonwealth Fund Dissemination Award 2015–2016 | 1 | $2,500 | 0.05% |
| Planning and Dissemination Grant– Institute Community Support | 16 | $184,183 | 1.14% |
| Patient Engagement | 3 | $35,000 | 0.71% |
| Patient Engagement – Collaboration Grants | 3 | $35,000 | 0.71% |
| Grand Total | 106 | $4,900,519 | 100.00% |
Appendix 5: Details on Indicators for which Data will be Available in Future
| Outcome | Indicators | Reason not available in 2015–2016 | Responsibility and expected timeline for initial reporting |
|---|---|---|---|
| 1) Building Capacity: Strengthened and expanded national ethics knowledge base | a) Number of ethics researchers funded vs CIHR and by program | These measures seek to examine all CIHR funding opportunities, not just those opportunities targeting ethics. As a result, a keyword methodology must be developed, tested and validated. The keyword method is being developed to identify:
|
Ethics Office Expected to be able to report on 2015–2016 data in 2016–2017 |
| 2) Building Capacity: Strengthened ethics research community in Canada | a) Percentage of ethics applications overall (success rates) | ||
| b) Number of grants sent to ethics reviewers (workload / burden / capacity) c) Number of applications ethics reviewers have received d) Number of ethics reviewers who were deployed to review an application |
Once we have a list of “ethics” reviewers (developed using a keyword methodology- see above), we can collect these data. The keyword approach will help us identify who these individuals are, beyond the holders of grants designated as ethics grants. | Ethics Office, in collaboration with the College of Reviewers with respect to reviewer-related data 2016–2017 | |
| e) Number of ethics reviewers recruited in the College of Reviewers | Recruitment of reviewers into the College is upcoming. | Ethics Office, in liaison with the College of Reviewers Branch 2017–2018 | |
| f) Percentage of targeted funding opportunities with an ethics component that lead to ethics researchers being funded as principal investigators or co-investigators | Funding release dates for funding opportunities launched in 2015–2016 are spread across 2015–2016 and 2016–2017, so a full picture of ethics researchers funded through relevant targeted funding opportunities is not yet available. Also, a method to identify ethics researchers is being developed. | Ethics OfficeFull results on 2015–2016 data available in 2016–2017 | |
| 3) Advancing Knowledge: Strengthened and expanded national ethics knowledge base | a) Percentage of ethics grants reporting new method, new theory or replication of findings | End-of-grant reports (RRS) are due from grant/award recipients within 18 months of the end of the funding period, and are not yet available for the validated grants and awards in 2015–2016. Previous years of “ethics” grants and award were not validated using our current methodology, so RRS reports from those grants cannot be used. | Performance and Accountability Branch, in collaboration with the Ethics Office 2017–2018 (partial data on 2015–2016 grants/awards). |
| b) Number of publications in ethics from CIHR-funded researchers c) Citations of findings of ethics research in scientific and non-scientific publications |
Once the keyword methodology is finalized, bibliometric analysis can be undertaken. | Performance and Accountability Branch in collaboration with the Ethics Office 2017–2018 | |
| 4) Informing decision-making: Ethics research that informs decision-making and practices in health and health research | a) Co-author analysis for ethics researchers being cited on publications b) Field analysis of citations of ethics research |
Once the keyword methodology is finalized, bibliometric analysis can be undertaken. | Performance and Accountability Branch, in collaboration with the Ethics Office 2017–2018 |
| c) Percentage of ethics grants reporting contribution to improved health of Canadians d) Percentage of ethics grants reporting application of findings e) Percentage of ethics grants reporting contribution to more effective health services and products |
End-of-grant reports are due from grant/award recipients within 18 months of the end of the funding period, and are not yet available for the validated grants and awards that were active in 2015–2016. Previous years of “ethics” grants and awards were not validated using our current methodology, so RRS reports from those grants cannot be used. | Performance and Accountability Branch, in collaboration with the Ethics Office 2017–2018 (partial data on 2015–2016 grants/awards). |
- Date modified: